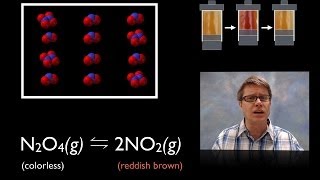
15.2 Le Chatelier's Principle | General Chemistry
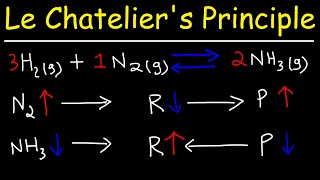
Chad provides a comprehensive lesson on Le Chatelier's Principle which states that if a stress is placed on a system at equilibrium, the system will respond to counteract the stress. If you're at all struggling with this concept, then check out this lesson as Chad explains Le Chatelier's Principle from 3 different perspectives: based upon the definition, by comparing the reaction quotient (Q) to the equilibrium constant (K), and by comparing the forward rate to the reverse rate. They all reinforce each other to help the student correctly predict whether a system will shift left or shift right including how to predict temperature and pressure effects.
I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at https://www.chadsprep.com/chadsgener...
If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at https://www.chadsprep.com/genchemyou...
00:00 Lesson Introduction
00:28 Introduction to Le Chatelier's Principle
01:10 The Reactions Quotient and Comparing Q to K
06:14 Adding a Reactant (Shift Right)
09:21 Removing a Product (Shift Right)
12:15 Adding a Solid (No Shift)
13:41 Changing the Temperature
17:52 Changing the Pressure
21:24 Adding an Inert Gas
https://www.chadsprep.com/
https://courses.chadsprep.com/pages/p...





![The moment we stopped understanding AI [AlexNet]](https://i.ytimg.com/vi/UZDiGooFs54/mqdefault.jpg)


![Data Modeling for Power BI [Full Course]](https://i.ytimg.com/vi/MrLnibFTtbA/mqdefault.jpg)