16.2a Pi Molecular Orbitals of 13 Butadiene | Organic Chemistry
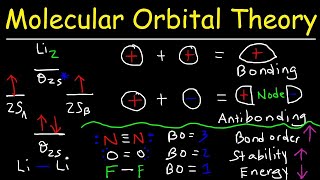
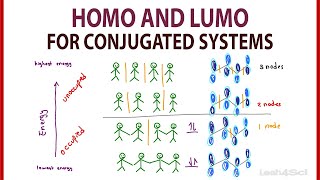
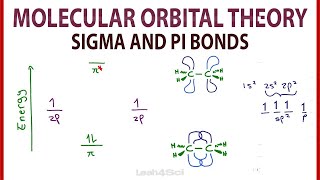
Chad provides a comprehensive introduction to Pi Molecular Orbitals for conjugated systems. He begins with a review of the pi molecular orbitals of ethylene. He demonstrates how inphase overlap leads to the formation of a lower energy bonding molecular orbital, while outofphase overlap creates a node resulting in the formation of a higher energy antibonding molecular orbital. Chad then applies these principles to draw the pi molecular orbitals of 1,3butadiene, a conjugated diene. He shows its molecular orbital diagram including illustrations of all four pi molecular orbitals and identifies which are bonding and antibonding and highlights the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO), collectively referred to as the Frontier Molecular Orbitals. Chad also describes the pattern for the number of vertical nodes in each molecular orbital and the alternating pattern for which orbitals or symmetric and antisymmetric.
If you want all my study guides, quizzes, and practice exams, check out my premium course at https://www.chadsprep.com/organicche...
00:00 Lesson Introduction
00:35 Review of the Pi Molecular Orbitals of Ethylene
10:30 Pi Molecular Orbitals of 1,3Butadiene
https://www.chadsprep.com/