19.2 How to Balance Redox Reactions (Half-Reaction Method) | General Chemistry
Chad provides a detailed lesson on how to balance redox reactions (i.e. oxidationreduction reactions) specifically using the halfreaction method. The lesson begins with an introduction to redox reactions showing how to identify the species being oxidized and the species being reduced and then the reducing agent (aka reductant) and oxidizing agent (oxidant). Two examples of balancing redox reactions are then worked out. The first doesn't have any H+ in the net reaction and so there ends up being no difference between balancing it under acidic conditions vs basic conditions. But the second is balanced both under acidic conditions (the default) and basic conditions. Chad demonstrates that the difference between the two involves neutralizing all the H+ in the reaction.
I've embedded this playlist as a course on my website with all the lessons organized by chapter in a collapsible menu and much of the content from the study guide included on the page. Check this lesson out at https://www.chadsprep.com/chadsgener...
If you want all my study guides, quizzes, final exam reviews, and practice exams, check out my General Chemistry Master Course (free trial available) at https://www.chadsprep.com/genchemyou...
00:00 Lesson Introduction
00:27 Introduction to Redox Reactions
05:17 How to Identify Oxidizing and Reducing Agents
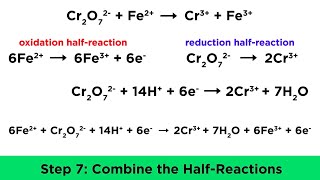
09:08 How to Balance Redox Reactions in Acidic Solution Example #1
21:17 How to Balance Redox Reactions in Acidic Solution Example #2
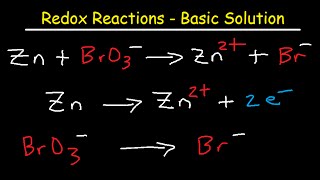
27:17 How to Balance Redox Reactions in Basic Solution
https://www.chadsprep.com/
https://courses.chadsprep.com/pages/p...