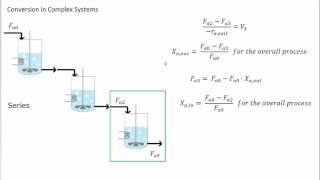
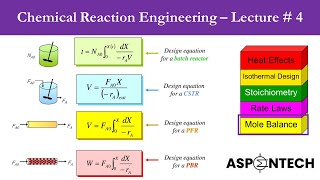
8) Example Problem Calculate Reactor Volume for CSTR PFR and time for batch reactor
In this video I solve the following problem (115) from Elements of Chemical Reaction Engineering, Fogler, 4th ed.
115) The reaction A B, is to be carried out isothermally in a continuous flow reactor. Calculate both the CSTR and PFR reactor volumes necessary to consume 99% of A (ie. C_A = 0.01C_Ao) when the entering molar flow rate is 5 mol/h, assuming the reaction rate r_A is:
a) r_A = k with k = 0.05 mol/ (h*dm^3)
b) r_A = kC_A with k = 0.0001 s^(1)
c) r_A = kC_A^2 with k = 3 dm^3 / (mol*h)
The entering volumetric flow rate is 10 dm^3/h. (note: F_A = C_A*v. For a constant volumentric flow rate v=v_o, then F_A = C_A*v_o. Also, C_Ao = F_Ao/v_o = [5 mol/h]/[10 dm^3/h] = 0.5 mol/dm^3)
d) Repeat (a), (b), and (c) to calculate the time necessary to consume 99.9% of species A in a 1000 dm^3 constant volume batch reactor with C_Ao = 0.5 mol/dm^3.