Aniline Synthesis via Tin Reduction (Science Explained)
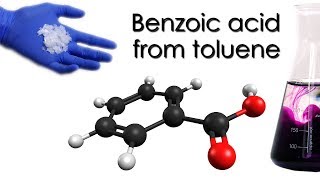
Welcome back, chemistry enthusiasts! Today, we're diving into a classic reaction in organic chemistry: the synthesis of aniline from nitrobenzene. In a previous video we produced nitrobenzene from benzene which was produced from benzoic acid made from toluene. Nitrobenzene is our starting point—a yellow, aromatic compound that's notorious for its toxicity but incredibly useful in synthesis. We'll be converting this into aniline, a key chemical in the manufacture of dyes, drugs, and polymers.
Links:
SUBSCRIBE ► https://www.youtube.com/c/WheelerScie...
Patreon ► patreon.com/user?u=86389980
Discord ► / discord
Instagram ► / wheelerscientific
eBay ► https://www.ebay.com/usr/wheelerscien...
References and uses:
The procedure was adapted from Adams, R., Johnson, J. R., & Wilcox, C. F. (1979). Laboratory experiments in Organic Chemistry. Macmillan.
Chapters:
00:00 Introduction
00:33 Reaction
03:34 Steam Distillation
04:17 Aniline Separation
05:01 Aniline Distilation
05:48 Analysis
Thanks for watching!