Atomic Number. Mass Number. Isotopes and Isobars
Concept of atomic number (Z), mass number (A), isotopes and isobars.
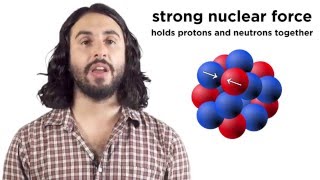
The atomic number is represented with the leter Z and is the number of protons that are at the nucleus of an atom. It defines the identity of a chemical element. Changing the number of protons of an element turns it into antoher one.
The mass number is the sum of particles at the nucleus of an atom, this is the sum of protons + neutrons. Mass number is represented with lhe letter A.knowing the atomic number (Z), the mass number (A) let us identify the different isotopes of a chemial element.
The isotopes are variants of a chemical element with different mass number, this is with different number of neutrons, because Z is the same.
On the othe hand we can have elements with different atomic number Z, but the same mass number A, these are called isobars, i.e. uranium238 and plutonium238 that have the same mass number, but of course, different atomic number, because they are different chemical elements.
On the other hand, the isotones (I don´t talk about the on the video) are the nuclides with the same number of neutrons and, of course, different number of protons.
https://en.wikipedia.org/wiki/Atomic_...
https://en.wikipedia.org/wiki/Mass_nu...
https://en.wikipedia.org/wiki/Isotope
https://en.wikipedia.org/wiki/Isobar_...)
https://en.wikipedia.org/wiki/Isotone
https://en.wikipedia.org/wiki/Atomic_...
The atomic number is the number of protons found in the nucleus of an atom. It is a fundamental property of an element and determines its unique identity in the periodic table. Elements are arranged in ascending order of atomic number on the periodic table, and each element has a distinct atomic number. The atomic number also determines the number of electrons in a neutral atom of the element. This value is critical in chemistry for understanding the organization of elements and predicting their chemical properties based on their atomic structure.
The mass number of an atom is the total number of protons and neutrons found in its nucleus. It is a whole number and is denoted by the symbol
A. The mass number is used to differentiate between isotopes of an element, which have the same atomic number (number of protons) but different numbers of neutrons. Isotopes of an element have the same chemical properties but may have different physical properties due to differences in mass. The mass number is crucial in nuclear physics for understanding nuclear structure and stability, as well as in chemistry for calculating the atomic mass of elements.
Isotopes are variants of a chemical element that have the same number of protons in their atomic nuclei but differ in the number of neutrons. This variation in neutron number leads to differences in mass number among isotopes of the same element. Isotopes of an element exhibit similar chemical properties but may have different physical properties due to variations in mass. Isotopes are commonly represented using the element's symbol followed by a hyphen and the mass number (e.g., carbon12, carbon13).
Isobars in the context of nuclides refer to atomic nuclei that have the same mass number but different atomic numbers. These nuclides occupy different positions on the periodic table but share the same total number of nucleons (protons and neutrons). Isobars may exhibit different chemical properties due to differences in atomic number, but they have identical mass numbers. This phenomenon is significant in nuclear physics, where isobars are studied to understand nuclear structure, stability, and reactions. Isobars also have practical applications in fields such as nuclear energy, radiology, and nuclear medicine.
Patreon ► / cienciabit
Susbcribe ► https://www.youtube.com/subscription_...
Do you want to contribute subtitles? ► http://www.youtube.com/timedtext_cs_p...
Cienciabit ► http://www.cienciabit.com
Thanks to Cienciabit's Patreon patrons:
Eduardo Pérez Rodríguez
Marc Juan
Wilson Caballero