Close Packed Structures || The Solid State - 11 || Chemistry Class 12 in Hindi
In this Chemistry video in Hindi for Class 12 we explained the structures of different close packed structures in solid crystals. This is a topic from the chapter 'The Solid States' for Class 12th Chemistry.
The constituent particles in solids are closely packed and create different 3 dimensional structures.
1. Close packing in 2 dimensions
a) Square close packing
b) Hexagonal close packing
2. Close packing in 3 dimensions
a) Simple Cubic close packing
b) Hexagonal close packing
c) Face Centred Cubic close packing
Three dimensional close packing from two dimensional hexagonal close packed layers:
Let us take a two dimensional hexagonal close packed layer ‘A’ and place a similar layer above it such that the spheres of the second layer are placed in the depressions of the first layer. Since the spheres of the two layers are aligned differently, let us call the second layer as B.
It can be observed that all the triangular voids of the first layer are not covered by the spheres of the second layer. This gives rise to different arrangements. Wherever a sphere of the second layer is above the void of the first layer (or vice versa) a tetrahedral void is formed. These voids are called tetrahedral voids because a tetrahedron is formed when the centres of these four spheres are joined.
At other places, the triangular voids in the second layer are above the triangular voids in the first layer, and the triangular shapes of these do not overlap. One of them has the apex of the triangle pointing upwards and the other downwards. Such voids are surrounded by six spheres and are called octahedral voids.
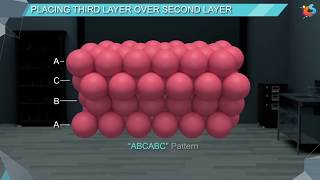
When third layer is placed over the second, there are two possibilities.
(i) Covering Tetrahedral Voids: Tetrahedral voids of the second layer may be covered by the spheres of the third layer. In this case, the spheres of the third layer are exactly aligned with those of the first layer. Thus, the pattern of spheres is repeated in alternate layers. This pattern is often written as ABAB ....... pattern. This structure is called hexagonal close packed (hcp) structure.
(ii) Covering Octahedral Voids: The third layer may be placed above the second layer in a manner such that its spheres cover the octahedral voids. When placed in this manner, the spheres of the third layer are not aligned with
those of either the first or the second layer. This arrangement is called ‘C’ type. Only when fourth layer is placed, its spheres are aligned with those of the first layer. This pattern of layers is often written as ABCABC ........... This structure is called cubic close packed (ccp) or facecentred cubic (fcc) structure.
THE SOLID STATE FULL CHAPTER Class 12 Chemistry
• Chemistry 12th : The Solid State
Previous videos :
The Solid State
• The Solid State (Introduction) || Che...
Types of Crystalline Solids
• Types of Crystalline Solids (The Soli...
NON POLAR molecular solids and LONDON dispersion forces
• NON POLAR molecular solids and LONDON...
Polar Molecular Solids and Hydrogen Bonded Molecular Solids
• Video
Ionic Solids
• Ionic Solids (The Solid State 5) ||...
Metallic Solids
• Metallic Solids (The Solid State 6)...
Covalent or Network Solids
• Covalent or Network Solids (The Solid...
Unit Cell and Packing Efficiency
• Unit Cell and Packing Efficiency (The...
Calculations involving Unit Cell Dimensions
• Calculations involving Unit Cell Dime...


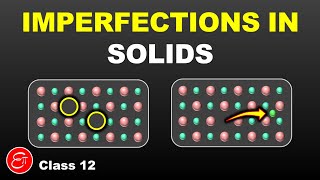












![Defects in Solids [Complete] in 25 minutes | Solid State](https://i.ytimg.com/vi/QaC-phLCXQw/mqdefault.jpg)








