de Broglie's Explanation of Bohr's Second postulate of Quantization Part 1
For more information:
http://www.7activestudio.com
[email protected]
http://www.7activemedical.com/
[email protected]
http://www.sciencetuts.com/
[email protected]
Contact: +91 9700061777,
04064501777 / 65864777
7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital content provider for K12. We also customise the content as per your requirement for companies platform providers colleges etc . 7 Active driving force "The Joy of Happy Learning" is what makes difference from other digital content providers. We consider Student needs, Lecturer needs and College needs in designing the 3D & 2D Animated Video Lectures. We are carrying a huge 3D Digital Library ready to use.
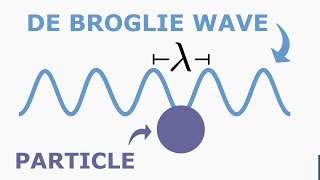
De Broglie's Explanation of Bohr's Second postulate of Quantization:It is very difficult to explain the Bohr's 2nd postulate. It states that an electron can revolve only in those orbits for which its orbital angular momentum Is an integral multiple of times when n =1,2,3, the quantization is why should the angular momentum can have only those values that are integral multiple of The question was explained by the French physicist Louts de Broglie in 1923, ten years after Bohr's proposal.According to De Broglie's hypothesis all material particles, such as electrons, also have a wave nature.C.J.Davisson and LH Germer Later experimentally verified the wave nature of electrons in 1927. De Broglie argued that the electron in its Circular orbit must be seen as a particle wave. In analogy to waves travelling on a string, particle waves to can lead to standing waves under resonant conditions.We know that when a string is plucked, a vast number of wave lengths are excited. However only those wave lengths survive which have nodes at the ends and form the standing wave in the string. In a string, standing Waves are formed when the total distance travelled by a wave down the string and back is one wavelength, two wavelengths, or any integral number of wavelengths. Waves with other wavelengths interfere with themselves upon reflection and their amplitudes quickly drop to zero. For an electron moving is nth circular orbit of radius rn, the total distance is the circumference of the orbit, 2πrn. A standing wave is shown on a circular orbit where four de Broglie wavelengths fit into the circumference of the orbit. 2πrn=nλ Equation n = 1,2,3 so on.
Figure Illustrates a standing particle wave or a circular orbit for n = 4,I.e., 2πrn = 4λ, where λ is the deBroglie wavelength of the electron moving in nth orbit. And we know. that λ where P is the momentum. If the speed of the electron is much less than the speed of the light then momentum p = mvn