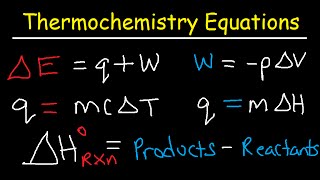Deriving the Thermodynamic Master Equations (Chemistry/Physics)
Combining the 1st and 2nd Laws of Thermodynamics, and then introducing enthalpy and the Gibbs energy. This is either Chemistry or Physics or just science really
#chemistry #physics #thermodynamics #stem #enthalpy #entropy #science #education
Throughout this analysis, I will be considering thermodynamic closed systems, in the sense that particles neither enter or leave a system. The internal energy of a closed system can comprise of many components and often components of kinetic energy, whether that be translational, rotational or vibrational, intermolecular interactions (often described by "intermolecular forces" (IMF) such as London dispersion forces or hydrogen bonds), covalent bond energies, or lattice energies. Defining the internal energy of a bulk system is a standard practice in classical thermodynamics to help us not have to keep track of what every single particle or object is doing when we do something to the closed system. Classical thermodynamics turns out to be a fantastic way of analysing bulk properties of materials and the world around us without even needing to appeal to quantum mechanics. indeed these principles were defined before the quantum world even started to be properly explored and appreciated in the 20th century. Wrapping up the behaviour of systems of particles on bulk scales introduces the state functions of enthalpy, entropy and Gibbs energy, amongst others that can be defined depending on circumstance such as the Helmholtz energy, as ways of relating almost abstract concepts of energy and its distribution (or spreading outness) to directly measurable quantities that can be determined in simple laboratory experiments.
A key mathematical idea explored in this video is using the concept of a differential form of a function. This is a way of representing infinitesimal changes in quantities in terms of others. This is a key extrapolation of high school calculus towards considering the mathematics of change of functions of multiple variables. In thermodynamics and chemistry, these quantities includes things like pressure, temperature and volume; even the simple ideal gas equation indicates that these physical quantities are properly interlinked.
So starting with a definition of an infinitesimal change in internal energy as only coming from small amounts of heat and work being done on a closed system, the Master Equations seek to link this fundamental principle of energy conservation (the First Law of Thermodynamics) to macroscopic properties and observations. The first Master Equation combines the First Law with the Second Law of Thermodynamics together in one expression, linking internal energy directly to both physical properties and entropy. Entropy being essentially a measure of a spread for energy e.g. a very localised picture of energy corresponds to a low entropy.
Defining enthalpy (H) and a new state function, cunningly defined as H = U +pV, can link these combined First and Second Laws of Thermodynamics to the measurable heat change observed in a laboratory experiment. Using a constant pressure situation as is common for bench chemistry, the (reversible) heat released from a reaction, which is measured with a thermometer monitoring a temperature variation, links directly to an enthalpy change. This analysis in turn can allow a scientist to measure the entropy change of a system. Being able to determine changes in these state functions from direct measurements goes a long way to help a chemist predict whether a chemical process will "go" (be feasible or "spontaneous" assuming any kinetic barrier can be overcome). Beyond that, the smart chemist can then work out how to tweak experimental conditions to ensure a desirable outcome from a planned reaction.
A third Master Equation can be derived that links thermodynamic state functions and physical quantities in a different way, and indeed in a really practical way. This involves introducing the new state function of the Gibbs Energy (G), sometimes called the Gibbs Free Energy, defined as G = H TS. The infinitesimal change in Gibbs Energy incorporates the previous results from the second Master equation that concerns enthalpy change to get a differential relationship to infinitesimal changes in only physical quantities, specifically changes in pressure and changes in volume. Given that the Gibbs energy has a direct link to the entropy change of the universe, this differential form can be used to apply the Second Law of Thermodynamics directly to experimental data.
These types of considerations of mathematical differential forms can be key in science education to understanding multivariate calculus a really powerful tool in both chemistry and physics and many more ways of modelling the real world. These differential forms link directly to ideas of partial differentiation, which will always be required when functions depend on multiple variables this is an unavoidable maths upgrade needed for all budding scientists.