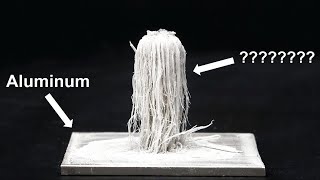
Dissolving an Aluminum Can in CuSO4
The reaction of Copper (II) sulfate with an Aluminum can results in the entire can dissolving. It is a popular chemistry demonstration. The chemical reaction in the video took place over a 24 hour time period, although the reaction starts immediately on adding the Aluminum can to the CuSO4 solution.
Note that the CuSO4 used in this video is in the hydrated form (CuSO4 . 5H2O). Anhydrous CuSO4 would work as well. In addition, note that the chemical reactions in this video are not balanced.
In order for this reaction to take place quickly it is necessary to at Sodium chloride (NaCl) to the CuSO4 (aq) solution. This destabilizes the thin layer of Al2O3 on the outside of the Al can and creates “pits” where the reaction can take place.
You may have also noticed bubbles in the video. The reaction of Al and CuSO4 doesn’t explain their presence. There is no gas formed in the equation below:
Al (s) + CuSO4 (aq) = Al2(SO4)3 (aq) + Cu (s)
Normally Aluminum metal is coated with a think layer of Aluminum oxide (Al2O3). This prevents water (and CuSO4) from reaching the pure Al metal. A small amount of Sodium chloride (NaCl) was added earlier and this destabilized the Al2O3 layer and allowed both water and CuSO4 to reach the Al (s).
In addition to the Al and CuSO4 reacting, the Al will also react with water:
Al + H2O = Al(OH)3 + H2
The H2 is a gas and those are the bubbles we see.
The substances used in this video are generally safe. The Al can, and the NaCl, aren’t toxic. The CuSO4 is commonly sold in stores and online to treat pipes for tree roots. “Copper sulfate is only moderately toxic upon acute oral exposure” according to http://pmep.cce.cornell.edu/profiles/...
Resources for Al + CuSO4 = Al2(SO4)3 + Cu:
Balanced Equation: • Balance Al + CuSO4 = Cu + Al2(SO4)3 ...
Net Ionic Equation: • How to Write the Net Ionic Equation f...
Type of Reaction: • Type of Reaction for Al + CuSO4
Resources for Al + H2O = Al(OH)3 + H2:
Balanced Equation: • Balance Al + H2O = Al(OH)3 + H2 (Alum...
The plastic liner on Aluminum cans has health implications. The liner releases Bisphenol A (BPA) which has been implicated in various health problems. It is a “a synthetic chemical with endocrine disrupting properties.” See https://www.ncbi.nlm.nih.gov/pmc/arti...