It was never so easy to get YouTube subscribers
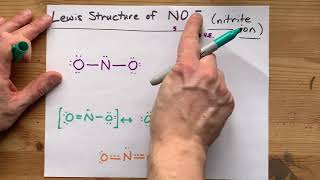
Draw the Lewis Structure of NO(+)
NO(+) is nitrogen monoxide without one of its electrons.
It has ten valence electrons total, which make it isoelectronic with nitrogen gas you probably already know this means there is a triple bond in between the two.
Check me out: http://www.chemistnate.com
Recommended