Get real, active and permanent YouTube subscribers
Enamine: Formation properties and Reactivity
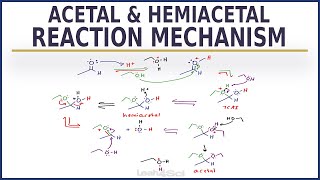
This lecture describes the formation of enamines by reaction of carbonyls with secondary amines. The mechanism is similar to Imine formation except that last deprotonation occurs at carbon since iminium ion doesn't contain any hydrogen attached to nitrogen.
Enamines beta carbon is more basic than its nitrogen due to pi donation by nitrogen lone pair into alkene pi bond. Enamine nitrogen is less basic than nitrogen of corresponding amine. Overall enamine is more basic than corresponding amine. And because of this basicity enamine shows many reactions at beta carbon with electrophiles like alkylation, conjugate addition and cross Aldol reaction etc.
Recommended