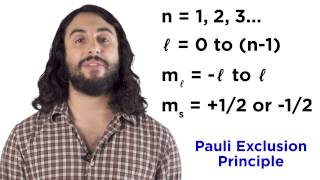Energy Levels Sublevels and Orbitals
Join this channel to get access to perks:
/ @joedelyncruz
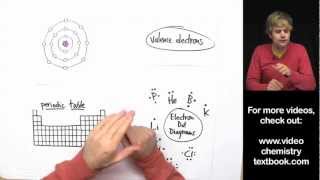
ENERGY LEVELS
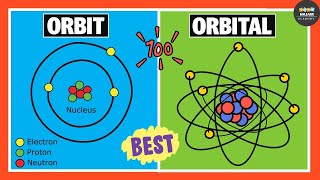
An atom has different energy levels which are represented by the symbol "n”. The energy levels are also known as electron shells or principal quantum levels. These energy levels are designated by the numbers 1, 2, 3, and so on.
In an atom, this is the 1st energy level or n=1. The first energy level is the closest to the nucleus and has the lowest energy. Electrons in the first energy level experience a stronger attraction to the positively charged nucleus, resulting in lower energy levels. This is the second energy level, 3rd, 4th and 5th. As the energy levels move higher (n=2, n=3, and so on), the electrons are farther from the nucleus and experience a weaker attraction, leading to higher energy levels.
Each energy level can accommodate a specific maximum number of electrons, and as you move to higher energy levels, the capacity for electrons increases.
SUBLEVELS
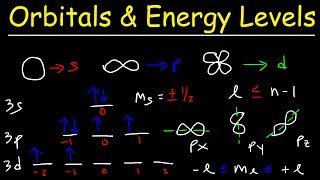
The Sublevels are also known as subshells. These refer to the different types of orbitals within an energy level where electrons are likely to be found. The sublevels (or subshells) are labeled with letters: s, p, d, and f, and each has a distinct shape and orientation.
As mentioned earlier, the sublevels are expressed as s, p, d, f, and g.
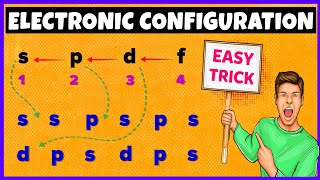
In the first energy level, there is only 1 sublevel, which is s. The s sublevel is also present in the second, third, fourth, and fifth energy levels. In the second energy level, there are 2 sublevels: s and p. The p sublevel can also be found in the third, fourth, and fifth energy levels. In the third energy level, there are 3 sublevels: s, p, and d. The d sublevel is also existing in the fourth and fifth energy levels. In the fourth energy level, there are 4 sublevels: s, p, d, and f. The f sublevel is also spotted in the fifth energy level. In the 5th energy level, there are 5 sublevels: s, p, d, f, and g.
ORBITALS
Sublevels contain orbitals. Orbitals are specific regions of space where electrons are likely to be found.
Each sublevel consists of one or more orbitals. For the s sublevel, there is one orbital. For the p sublevel, there are 3 orbitals. For the d sublevel, there are 5 orbitals. For the f sublevel, there are 7 orbitals. For the g sublevel, there are 9 orbitals. Each orbital has a maximum of 2 electrons, which spin in different directions—one clockwise and the other counterclockwise. The maximum number of electrons in the s orbital is 2, in the p orbital is 6, in the d orbital is 10, in the f orbital is 14, and in the g orbital is 18 electrons.
Contact: [email protected]