🔬 Ethanol-Water Distillation: Breaking It Down in Simple Terms! 🎯
Receive Comprehensive Mathematics Practice Papers Weekly for FREE
Click this link to get: ▶▶▶ https://iitutor.com/emaillist/ ◀◀◀
Unlock the magic of EthanolWater Distillation!
Have you ever wondered how we separate ethanol and water to harness their incredible properties? This video breaks down the science of EthanolWater Distillation in simple terms.
Learn the secrets of why ethanol and water love to mix, the industrial significance of ethanol, and how you can renewably produce it through fermentation. But the real challenge lies in separating these two inseparable pals!
Discover the stepbystep method and equipment you need to conduct this fascinating experiment. We'll show you how to set up the apparatus, heat it gently, and collect those precious distillates.
Get ready for a handson journey into chemistry, where we explore the impacts of hydrogen bonding and the density differences that influence the distillation process.
Brace yourself for a whirlwind of knowledge and watch as we demystify the science behind EthanolWater Distillation. Join us in unravelling the secrets of this captivating process! ✨
Motivation
Ethanol and water are extremely soluble in one another.
Ethanol is used heavily in industry.
It can be produced renewably from fermentation at concentrations of 15% v/v.
It must be separated from the water to be used effectively.
Method
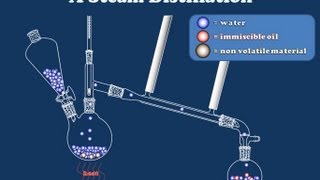
Set up the equipment as shown.
Place 100 mL of 20:80 ethanol/water mixture into the distillation flask.
Heat gently, the temperature of the water bath should be kept.
Collect 20 mL of distillate.
Typical results
Distillation will never achieve 100% purity.
Strong hydrogen bonding forces some water out with the ethanol.
Distillates with higher ethanol percentages will weigh less per mL.
This is due to ethanol’s lower density.

















![What are States of Matter in Chemistry? Solid Liquid Gas Plasma [112]](https://i.ytimg.com/vi/x2t6sUjL0T4/mqdefault.jpg)









