Explain the Counter Current Extraction? | Solvent Extraction | Analytical Chemstry
Download pdf notes at http://edmerls.com/explainthecounte...
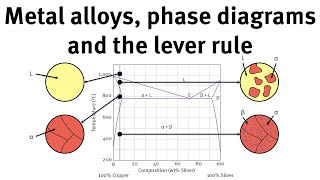
The method was discovered by Craig and Post. It is consists of 300400 such chambers. The organic solvent and the aqueous solution is introduced to tube A and then it passes to B. It is shaken and is allowed to attain the equilibrium. Now, the apparatus is tilted so that the upper layer gets decanted through C and is collected in D.
When the apparatus is again made vertical. The liquid passes through D into E in the next chamber of A and then to B. The process is repeated till the two liquids gets almost separated.
1. With the help of this method we can have accurate quantitative analysis of a single as well as the mixture of the components.
2. In this case apparatus required are very simple (separating funnel burette pipets conical flask etc.)
3. Time required for analysis is very small.
4. The method is very well used for detection of traces quantity of substance where precipitation method (Gravimetry) is not possible.
5. Fe+3 ferric ion can be easily extracted by ether from 6 molar HCl solution of the ferrous alloy and iron ore.
6. The extraction can also be used in the extraction of metal as metal chelate where chelate has high solubility in an immiscible solvent such as chloroform and benzene.
7. In industrial and commercial field extraction by counter current extraction is frequently applied in the separation of components where the difference in the distribution coefficient are small.
8. The phenomenon is widely applied in drug analysis.
9. The solvent extraction is used in clinical laboratory.
10. Metal chelates are more soluble in nonpolar solvents. Thus Ni(II) in its tetra coordinate complex with dimethyl glyoxime can be extracted into chloroform. In presence of citrate or tartrate the precipitation of Fe(III) and Cr(III) can be avoided.
Analytical Reasoning
• Solve Problems Related To Ages With T...
English Grammar
• Making Sense With Tense #1 English ...
Interview Skills
• Questions You May Be Asked During an ...
Managerial Economics
• MCQ #1 of Managerial Economics
Royalty Free Stock Footage
• Flowers Royalty free footage
Chemical Thermodynamics Physical Chemistry
• State and Explain first law of thermo...
Ionic Equilibria Physical Chemistry
• Derive expression for Ostwald’s Dilut...
Electrochemistry Physical Chemistry
• What are different types of Reversibl...
Solid State Physical Chemistry
• Explain the following terms | Solid S...
Gaseous State Physical Chemistry
• Postulates of Kinetic Molecular Theor...
Colloidal States Physical Chemistry
• What is Colloidal Solution? | Colloid...
Stereochemistry Organic Chemistry
• Explain Configuration and Conformatio...
Nanomaterials Engineering Chemistry
• Compare top down with bottom up Proce...
Water and Its Treatment Engineering Chemistry
• Explain why hard water gives out a cu...
Electrochemistry Engineering Chemistry
• Distinguish between metallic and elec...
Environmental Studies
• MCQ on Environmental Studies Part 8
Optics Applied Physics
• What are cartesian sign conventions f...
For Details Visit
http://cepekmedia.co.nf
http://cepek.hol.es/
http://edmerls.66Ghz.com/
http://edmerls.tk/