Explain the Role of complexing Agent in Solvent Extraction? | Solvent Extracton | Analytical
Download the pdf notes at http://edmerls.com/explaintheroleo...
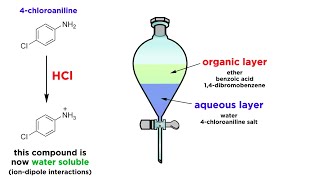
In general, organic solute (covalent solute) are more soluble in non polar (organic) solvents than water and hydrated salts are more soluble in water (polar) solvent. The inorganic salts in the solution are in the form of ions. In solvent extraction, we must see that solubility of inorganic salts in water should be less and solubility in organic solvent must be increased. The solubility of the metal ions in water is due to the association of water molecules with charged metal ions. in other words due to the hydration. Therefore, if the charged on the metal ions is neutralised, the solubility of the metal ions in the watt will be decreased. The charge on the metal ion is neutralised by reaction of metal ions with the appropriate complexing agent to form the metalchellate complex. The bulkier the complex and if it is more hydrophobic in nature better will be extraction. .. Each ion will have to be treated differently. Thus, aluminium ions in the solution are usually converted into hydroxy aluminium quinolate by the addition of 8hydroxy quinolene. The chellate thus formed can be extracted by using benzene or chloroform. It is possible to extract aluminium at pH 5:18, 3 in the presence of iron Nickel and Vanadium. The other complexing agents are dimethyl glyoxime, acetyl acetone cupferron, nitroso β naphthol dithizone etc.
#SolventExtraction #AnalyticalChemistry #Extraction #ComplexingAgent
Analytical Reasoning
• Solve Problems Related To Ages With T...
English Grammar
• Making Sense With Tense #1 English ...
Interview Skills
• Questions You May Be Asked During an ...
Managerial Economics
• MCQ #1 of Managerial Economics
Royalty Free Stock Footage
• Flowers Royalty free footage
Chemical Thermodynamics Physical Chemistry
• State and Explain first law of thermo...
Ionic Equilibria Physical Chemistry
• Derive expression for Ostwald’s Dilut...
Electrochemistry Physical Chemistry
• What are different types of Reversibl...
Solid State Physical Chemistry
• Explain the following terms | Solid S...
Gaseous State Physical Chemistry
• Postulates of Kinetic Molecular Theor...
Colloidal States Physical Chemistry
• What is Colloidal Solution? | Colloid...
Stereochemistry Organic Chemistry
• Explain Configuration and Conformatio...
Nanomaterials Engineering Chemistry
• Compare top down with bottom up Proce...
Water and Its Treatment Engineering Chemistry
• Explain why hard water gives out a cu...
Electrochemistry Engineering Chemistry
• Distinguish between metallic and elec...
Environmental Studies
• MCQ on Environmental Studies Part 8
Optics Applied Physics
• What are cartesian sign conventions f...
For Details Visit
http://cepekmedia.co.nf
http://cepek.hol.es/
http://edmerls.66Ghz.com/
http://edmerls.tk/