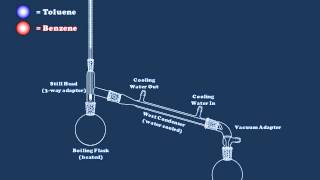
Fractional Distillation: Ethanol/Water Separation
In this demonstration, a mixture of ethanol and water is separated using fractional distillation.
This is a mixture of liquids with different boiling points: ethanol boils at 78.23 °C and water boils at 100 °C.
Sixth form students will be aware that evaporation occurs at all temperatures. In contrast to the simple explanation, a few water molecules do actually evaporate what the ethanol boils off at around 78 °C. It is therefore not a clean separation. A more complete separation would occur if the distillation was repeated. This can be made to occur by packing glass beads into a special compartment between the initial flask and the condenser. These beads can be seen in the video: the mixture condenses and boils on the surface of every bead on the way up to the condenser. This effectively means that the distillation is being repeated multiple times. Regardless of how many times the process is repeated, it is not possible to purify ethanol beyond 95.63% using fractional distillation. This mixture containing 95.63% ethanol is called an 'azeotrope'. The word 'azeotrope' is Greek for 'no change on boiling'. If 100% pure ethanol is required, then a molecular sieve can be used to remove the final water molecules. There are many types of molecular sieve: many are aluminosilicates. These beads contain tiny holes that can accommodate water molecules, leaving the 100% pure ethanol. The beads can be separated using filtration.