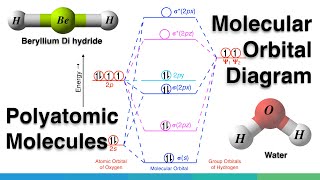
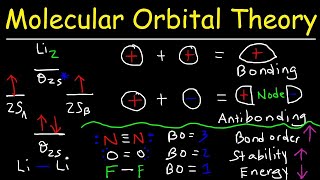
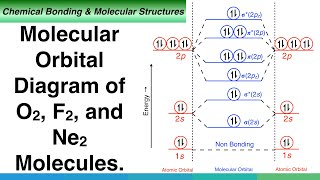
Gerade and Ungerade Molecular Orbitals. (SYMMETRY OF MOLECULAR ORBITALS)
The German words gerade means even and ungerade stands for uneven. They are used to describe the angular part of the wave functions for s, p and d orbitals.
When two S orbitals combine they form bonding and anti bonding orbitals as shown here. Please note that the sign indicated in molecular orbitals are not the sign of electron or proton. Because we are not talking about electron or proton, but we are only talking about orbitals so the sign indicated here is sign of wave function of orbital as we know that the crest of the wave is given with positive sign and trout is given with negative sign.
Also positive sign indicates the more probability of finding electron in that part of orbital while in negative part there is less probability.
Now if we try to find the molecular symmetry by dividing the molecular orbital from the center the bonding molecular orbitals are same on both sides as both are negative, So they are considered as geared means even. While in case of anti bonding molecular orbital when molecular orbital is divided from center one part is positive and other is negative so the anti bonding molecular orbital is ungerade means uneven.
They are represented by small letters g and u.
Similarly when 2p orbitals overlap to form sigma bonding and anti bonding molecular orbitals the shape of orbitals are as shown in diagram. Which can also be represented as waves like.
Now if we divide the molecule form center bonding moleculer orbital is geared as both side shows same sign of orbitals but anti bonding molecular orbitals are ungraded as both sides are having opposite signs, one is positive and the other is negative.
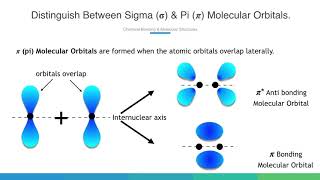
You can also check the symmetry of pi molecular orbital. In this case lateral overlap takes place and shape of orbital is different that sigma orbital. So we can divide the molecule at an angle to see the sign of molecular orbital if they are same or different. And it seems that the anti bonding molecular orbitals are symmetrical or grade while bonding molecular orbitals are unsymmetrical of ungerade.
Similar concept can also be applied to atomic orbitals like dxy and dz2 orbitals, Here both dxy and dz2 orbitals are geared.
Other Subjects:
Adult Education @ http://www.edmerls.com/index.php/Adul...
Analytical Chemistry @ http://www.edmerls.com/index.php/Anal...
Applied Physics @ http://www.edmerls.com/index.php/Appl...
Engineering Drawing @ http://www.edmerls.com/index.php/Engi...
English @ http://www.edmerls.com/index.php/Engl...
Environmental Studies @ http://www.edmerls.com/index.php/Envi...
General Medicine @ http://www.edmerls.com/index.php/Gene...
Mathematics @ http://www.edmerls.com/index.php/Math...
Organic Chemistry @ http://www.edmerls.com/index.php/Orga...
Physical Chemistry @ http://www.edmerls.com/index.php/Phys...
Patente B Italia @ http://www.edmerls.com/index.php/Pate...
Soft Skills @ http://www.edmerls.com/index.php/Soft...