15 Free YouTube subscribers for your channel
How to Prepare a Medical Device 510k Submission for FDA | Rob Packard | Joe Hage | Updated
https://MedicalDevicesGroup.net/Webin... for the slides.
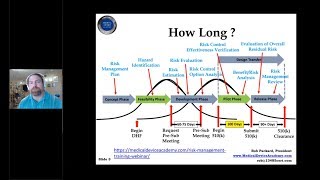
The Medical Devices Group presents Medical Device Academy founder Robert Packard. In an hourandahalf workshop, Rob covered Changes to RTA process, Human Factors Guidance, Changes to eCopy process, Small Business Qualification Changes, Interoperability Guidance, eSubmitter software status, Device Modification Guidances, Quik 510(k) Pilot, Impact of De Novo & Fee Changes, Cybersecurity Policies, UDI Requirements, New ISO 109931:2018 Standard.
Recommended