Hybridization of Atomic Orbitals | SP SP2 SP3 Hybridization of Carbon
This lecture is about hybridization of atomic orbitals, pi bonds, sigma bonds and sp, sp2, sp3 hybridization of carbon in chemistry.
Chemical Bonding Playlist:
• Chemical Bonding
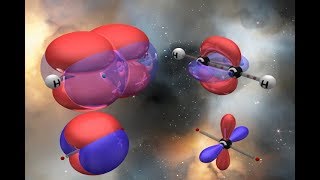
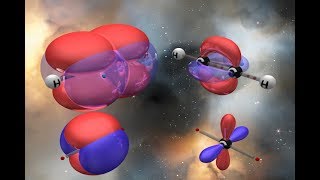
Q: What is hybridization of atomic orbitals?.
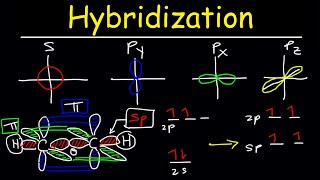
Ans: When orbitals of same or nearly same energy combine together to form new hybrid orbitals, this process is called hybridization.
For example,
When 1s orbital and 3p orbitals of a carbon atom combine together, they form sp3 hybridization.
When 1s orbital and 20 orbitals of a carbon atom combine together, they form sp2 hybridization.
When 1s and 1p orbitals of a carbon atom combine together, they form sp hybridization.
To learn more about hybridization of atomic orbitals, watch this lecture till the end.
#hybridizationofatomicorbitals
#spsp2sp3hybridization
#chemistry
#najamacademy
Subscribe my channel at: / @najamacademy
Youtube link: / @najamacademy
Facebook link: / najamacademy