Hybridization Theory (English)
Contents:
Chapter 1: Why Hybridization Theory was Developed, Why is it Important to Visualize Atoms within a Molecule in ThreeDimensions.
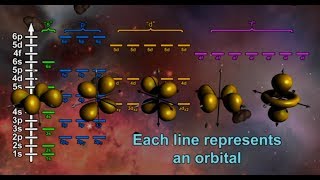
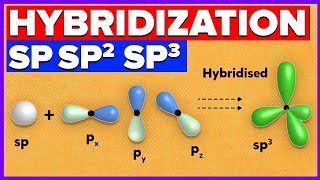
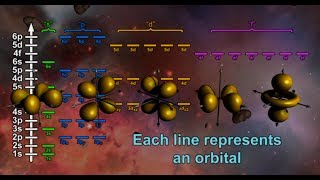
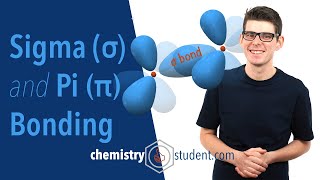
Chapter 2: Review of s and p Atomic Orbitals, How sp3 Carbon Formed, Shape of sp3 Orbitals and Angles, Representation on Blackboard or in Book, Sigma Bonds, Free Rotation of CC Sigma Bonds, Conformational Analysis, Energy of Eclipsed and Staggered Conformations, Newman Projections, Dihedral Angles, Barrier of Rotation.
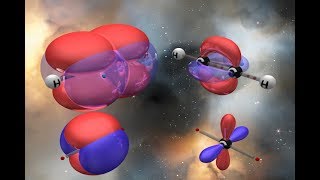
Chapter 3: How sp2 Carbon Formed, Shape of sp2 Orbitals and Angles, Representation on Blackboard or in Book, pi Bonds, Electron Density and Geometry of Double Bond, How to Draw Double Bonds in 3D, Geometric Isomers (E and Z), Isomerization of Geometric Isomers.
Chapter 4: How sp Carbon Formed, Shape of sp Orbitals and Angles, Representation on Blackboard or in Book, How to Draw Triple Bonds in 3D, pi Bonds, Electron Density and Geometry of Triple Bond.
Chapter 5: Deducing Hybridization of Atoms, Making the Leap from 2D to 3D via Hybridization Theory (Many Examples Worked for the Student), Heteroatom Hybridization, VSEPR, Deviations from Ideal Bond Angles, Nitrogen Inversion.
Narration: Gordon W. Gribble, PH.D., Dartmouth College
The DVD version is available at https://sponholtzproductions.com/