Ideal Gases vs. Real Gases
Need help preparing for the General Chemistry section of the MCAT? MedSchoolCoach expert, Ken Tao, will teach everything you need to know about ideal gases versus real gases. Watch this video to get all the mcat study tips you need to do well on this section of the exam!
Ideal gases do not exist in nature, but real gases approximate models of ideal gases under conditions of high temperature and low pressure. However, under conditions of low temperatures and/or high pressures, a real gas can no longer be treated as an ideal gas. In those cases, we need to have an understanding of how real gases behave. To understand the differences between ideal gases and real gases, we’ll compare the volume and pressure of each.
Volume
When considering ideal gases, the volume they occupy is simply the volume of the container. This is true because we assume that ideal gas particles have negligible volume. In contrast, real gases take up space, so the volume they occupy is less than the total volume of the container. The volume available for a real gas particle is equal to the total volume of the container minus the volume occupied by molecules of gas.
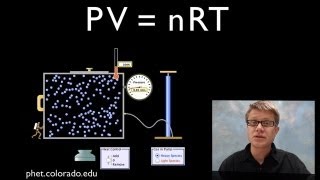
The ideal gas law equation (PV = nRT) doesn’t account for the reduced volume available to molecules of a real gas. Here is the ideal gas law with the corrections accounting for the volume of real gases shown, set equal to pressure:
For the volume available to real gases, you have to subtract the volume occupied by the gas molecules, equal to the product of n times b. The number of moles of gas is n, while b can be thought of as a constant that describes how large the molecules are. When you subtract the product of these two, you're able to subtract the volume occupied by the gas particles, correcting for the volume.
Pressure
With ideal gases, the gas particles move unencumbered and freely collide with the walls of the container to produce the pressure. This is true because ideal gas molecules do not experience intermolecular forces, as one of our assumptions for ideal gases states.
Conversely, real gas molecules do experience attractive intermolecular forces from one another, such that the gas particles don't move independent of one another. Consequently, the gas molecules have a tendency to clump in the middle and don't collide as frequently with the walls of the container. Therefore, real gases produce a lower pressure than ideal gases. Let’s revisit the Van Deer Waal’s equation, and how it corrects for the pressure of real gases. Below is our equation with corrections for volume, minus a new term.
The variable a is a constant representing the strength of the attractive forces. The variable n represents moles of gas, while v represents the volume. The relationship “n over v” is moles per volume, which expresses the concentration of gas particles in a container. The greater the concentration of gas particles, the more intermolecular interactions the gas molecules will have. A greater number of intermolecular interactions will lead to a reduced number of collisions, decreasing the total pressure. Stronger and more numerous intermolecular interactions will decrease the pressure of real gases, so an2/v2 is the correction for pressure. This equation can be rewritten into the typical form of the Van der Waals equation for real gases.
It is not recommended that you memorize the equations shown here. Instead, make sure you're able to understand why we corrected for the volume as well as why we corrected from the pressure for real gases in comparison to ideal gases.
MEDSCHOOLCOACH
To watch more MCAT video tutorials like this and have access to study scheduling, progress tracking, flashcard and question bank, download MCAT Prep by MedSchoolCoach
IOS Link: https://play.google.com/store/apps/de...
Apple Link: https://apps.apple.com/us/app/mcatpr...
#medschoolcoach #MCATprep #MCATstudytools








![Master the Ideal Gas Law in Chemistry A StepbyStep Guide [1510]](https://i.ytimg.com/vi/kZIpV4RcVZ0/mqdefault.jpg)