Free views, likes and subscribers at YouTube. Now!
Intermolecular Forces and Boiling Point (AP Chemistry)
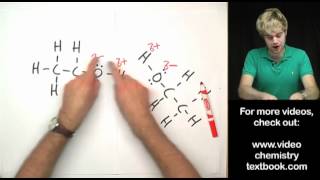
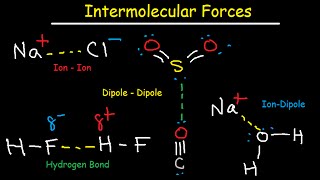
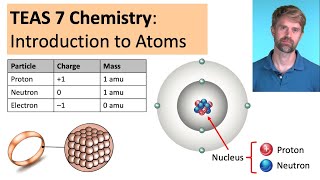
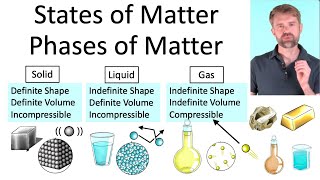
In this video, we look at an AP Chemistry multiple choice question (MCQ). We find a compound that has a boiling point similar to Argon. Boiling points are influenced by intermolecular forces or IMF's, so we need to figure out which of the answer choices has similar IMF's to Argon. Some of the key types of IMF's are hydrogen bonds, dipoledipole attractions, and London dispersion forces. London dispersion forces are caused by the random movement of electrons. This video is particularly relevant for AP Chemistry. It is also use for IB Chemistry, CBSE, NCERT, JEE, NEET, Alevel, IGCSE, and preparation for MCAT, SAT, DAT, and OAT tests.
Recommended