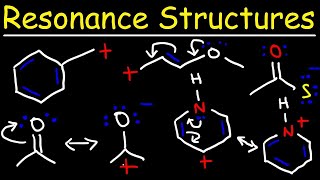
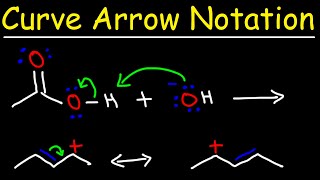
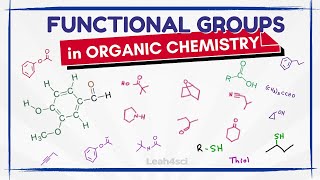
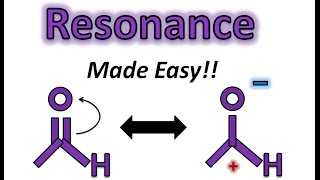
Introduction to the Curved Arrow Pushing Formalism in Organic Chemistry
To be successful in organic chemistry you need to be able to understand the flow of electrons. Since a pair of electrons forms a bond, it is important to know how electrons move from one reagent to the next. In this screencast we examine the concept of the curved arrow formalism to track how electrons move in a chemical reaction. A few general guidelines are helpful for understanding this convention. An arrow starts at a lone pair of electrons or a pi bond. The arrow ends at an positive charge or electron deficient atom or species. The arrow represents the movement of two electrons and the formation of a new bond. For every bond made, a bond has to be broken such that the convention of Lewis dot structures is obeyed. In any reaction, there is always an acidbase pair or more generally an electrophilenucleophile pair. Try to identify these before starting with your mechanistic proposal. This screencast examines a few different common reaction scenarios such as an acidbase reaction, ester saponification, and an SN2 reaction.