Ionic and Covalent Bonding | Chemical Bonding
Ionic and Covalent Bonds in Chemical Bonding
For Live Classes, Concept Videos, Quizzes, Mock Tests & Revision Notes please see our Website/App:
Our Website: http://bit.ly/2KBC0l1
Android App: https://bit.ly/3k48zdK
CBSE Class 11 Courses: https://bit.ly/48isN9Q
CBSE Class 10 Courses: https://bit.ly/363U55V
CBSE Class 9 Courses: https://bit.ly/39Pm7mM
CBSE Class 8 Courses: https://bit.ly/3bJByzB
ICSE Class 10 Courses: https://bit.ly/2MaXpFo
ICSE Class 9 Courses: https://bit.ly/3iFV7dl
ICSE Class 8 Courses: https://bit.ly/3boM5OB
IGCSE Courses: https://bit.ly/2YNwQcn
Artificial Intelligence: https://bit.ly/3vm3FAE
Python Coding: https://bit.ly/3nX0s2y
Java Coding: https://bit.ly/3chHTAK
Facebook page: http://bit.ly/2s6VYhf
Ionic and covalent bonds are two fundamental types of chemical bonds that hold atoms together to form molecules.
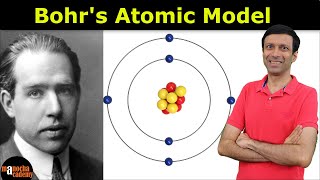
1. Ionic Bonds:
Ionic bonds form between atoms when there is a large difference in electronegativity, typically between a metal and a nonmetal.
In an ionic bond, one atom essentially donates an electron (or electrons) to another atom, resulting in the formation of positively charged ions (cations) and negatively charged ions (anions).
These oppositely charged ions are then attracted to each other due to electrostatic forces, forming an ionic compound.
Example: Sodium chloride (NaCl), where sodium (Na) donates an electron to chlorine (Cl), resulting in the formation of Na⁺ and Cl⁻ ions, which are attracted to each other to form the ionic compound sodium chloride.
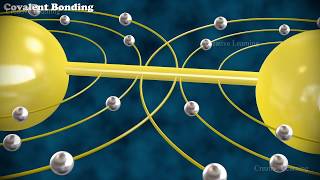
2. Covalent Bonds:
Covalent bonds form between atoms when they share one or more pairs of electrons.
Covalent bonds typically occur between nonmetal atoms with similar electronegativities.
In a covalent bond, each atom contributes one or more electrons to the shared pair, resulting in the formation of a molecule.
Covalent bonds can be further classified into single, double, or triple bonds based on the number of electron pairs shared between atoms.
Example: Hydrogen molecule (H₂), where each hydrogen atom shares a pair of electrons, forming a covalent bond and resulting in the stable molecule of hydrogen gas.
In summary, ionic bonds involve the transfer of electrons from one atom to another, resulting in the formation of ions, while covalent bonds involve the sharing of electrons between atoms to achieve a stable electron configuration.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!













![Chemical Formulas, Ionic & Covalent Bonds in Chemistry [1214]](https://i.ytimg.com/vi/puvMNEtfM9s/mqdefault.jpg)












