Lewis Diagrams Made Easy: How to Draw Lewis Dot Structures
Ketzbook demonstrates how to draw Lewis diagrams for elements and simple molecules using an easytofollow stepbystep explanation with several examples.
Get $300 free when you open a Chase checking account with this code:
https://accounts.chase.com/raf/invite...
Offer expires 10/16/2024
Want FREE stocks and the ability to trade stocks for free?
https://join.robinhood.com/benjamk94/
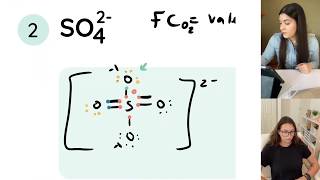
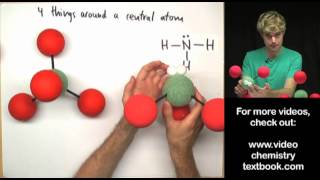
Lewis diagrams (aka Lewis structures, Lewis dot structures, Lewis dot diagrams) are useful because they use simple drawings to show how atoms share valence electrons in molecules, polyatomic ions, and other covalent structures. This is my first tutorial in the series.
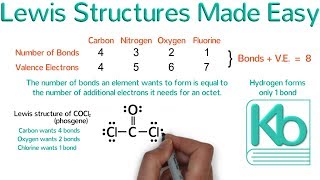
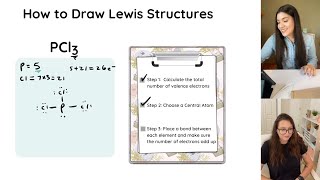
For simple molecules, follow these 5 steps:
1) count all the valence electrons
2) put the singular atom in the middle
3) draw in single bonds
4) put the remaining electrons in as lone pairs
5) give every atom an octet or duet by turning lone pairs into double or triple bonds as needed
Please also see the second video in my BONDING series:
• Lewis Structures Made Easy: Examples ...
Pocket Chemist: https://geniuslabgear.com/discount/KE...
Just in case you have seen the Lewis diagram of sulfur trioxide as something different (i.e. three double bonds as shown in Wikipedia or some old books), current theory suggests that the simple Lewis diagram I drew is most correct based on the lack of dorbital participation in bonding and the calculated charges (sulfur is positive and oxygens are negative as predicted from formal charges).
See:
(1) http://www.madsci.org/posts/archives/...
(2) Suidan, L., Badenhoop, J. K., Glendening, E. D., & Weinhold, F. (1995). Common Textbook and Teaching Misrepresentations of Lewis Structures. Journal of Chemical Education, 72(7), 583. https://pubs.acs.org/doi/epdf/10.1021...
(3) A. Reed and P.v.R. Schleyer, Journal of the American Chemical Society 109, 736273 (1987), ibid. 112, 143445 (1990); E. Magnusson, ibid. 794051. https://pubs.acs.org/doi/10.1021/ja00...
(4) Cunningham, Terence P.; Cooper, David L.; Gerratt, Joseph; Karadakov, Peter B. & Raimondi, Mario (1997). "Chemical bonding in oxofluorides of hypercoordinatesulfur". Journal of the Chemical Society, Faraday Transactions. 93 (13): 2247–2254. doi:10.1039/A700708F. https://pubs.rsc.org/en/Content/Artic...
(5) https://pubs.acs.org/doi/full/10.1021...
My goal is to make chemistry easier ;)
http://ketzbook.com
Donations are greatly appreciated and help make future videos possible:
Venmo: Benjamin Ketz @ketzbook
or Paypal: https://paypal.me/ketzbook?locale.x=e...
#chemistry #madeeasy #LewisStructures #ketzbook #tutorial #LewisDiagrams