Grow your YouTube channel like a PRO with a free tool
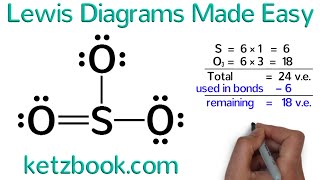
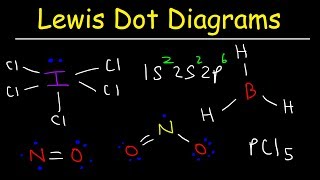
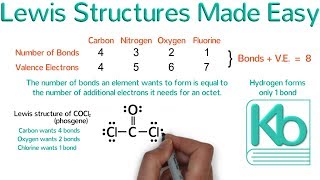
Lewis Structure of H2O water dihydrogen monoxide
Water has oxygen (a nonmetal) bonding with hydrogen (also nonmetals) and this means there is a SHARING of electrons (covalent bonding) between them.
Each hydrogen shares ONE electron with the oxygen, and oxygen shares ONE electron back with each of the hydrogens; this completes the outer shell of oxygen (it now has a complete octet) and hydrogen is satisfied as well (it obeys the "doublet rule" and not the octet rule, so it is happy with just two electrons).
Check me out: http://www.chemistnate.com
Recommended