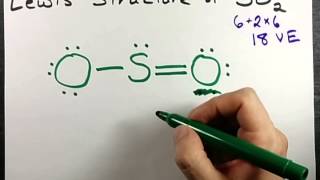Learn how to get Free YouTube subscribers, views and likes
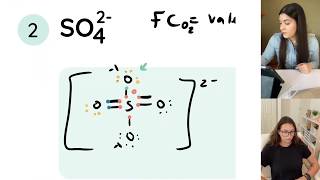
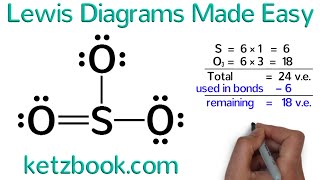
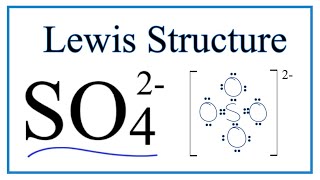
Lewis Structure of SO4(2-) (Sulfate) CORRECT
Sulfur make six bonds in this Lewis Structure. Two of the oxygens are singlebonded and two are doublebonded. The reason is FORMAL CHARGE and the fact that sulfur doesn't have to follow the octet rule. There is a "Resonance Structure" which you may (or may not) have to know about.
Check me out: http://www.chemistnate.com
Recommended