Lewis Structures for Polyatomic Ions
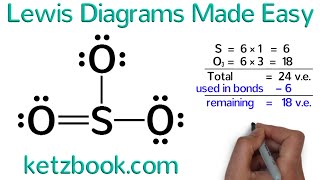
To draw Lewis Structures for polyatomic ions we’ll follow a few simple steps. We first count the number of valence electrons for the polyatomic ion.
Using the Periodic Table we find the number of valence electrons for each element. If the ion has a negative charge (like CN), we add an additional electron. If it has a positive charge (like NH4 +) we subtract an electron.
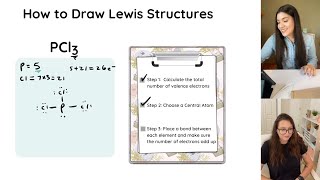
We then follow the general process for drawing Lewis Structures (tps:// • How to Draw Lewis Structures: Five Ea... ).
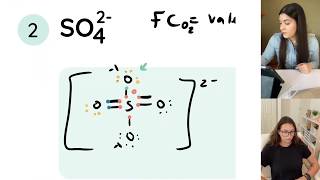
Not that for polyatomic ions we place brackets around the final Lewis Structure and place the charge on the ion outside of the brackets.
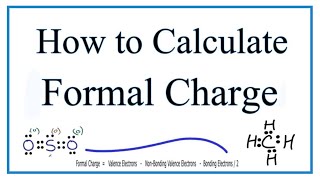
For more complicate Lewis Structures it is also a good idea to check the formal charges ( • Formal Charges: Calculating Formal Ch... ).
Helpful Videos:
How to Draw Lewis Structures: • How to Draw Lewis Structures: Five Ea...
Lewis Structures Practice Video Worksheet: • Lewis Dot Structure Practice Problems...
Determining Formal Charge: • Formal Charges: Calculating Formal Ch...
Formal Charge Practice Video: • Formal Charge Practice Problems with ...
Finding Valence Electrons (element): • Finding the Number of Valence Electro...
Finding Valence Electrons (molecule): • Finding the Number of Valence Electro...
The Octet Rule: • The Octet Rule: Help, Definition, and...
Exceptions to the Octet Rule: • Exceptions to the Octet Rule
Finally the Lewis Structure for SO4 2 is • How to Draw the Lewis Structure for t... .