Melanoma Immunotherapy with Dr. Jedd Wolchok
Dr. Jedd Wolchok leads a discussion on immunotherapy for melanoma, including new cancer research and treatments. #CRIsummit #immunotherapy #melnm
https://www.cancerresearch.org/virtua...
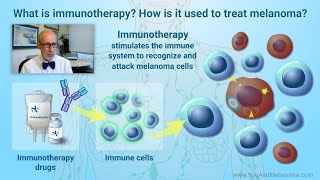
Immunotherapy has forever changed the way melanoma is treated. Checkpoint inhibitors, beginning with the landmark 2011 FDA approval of ipilimumab (Yervoy®), are responsible for the increasing survival rate for patients with metastatic melanoma. Nowcientists and clinicians are working to bring the benefits of immunotherapy to more patients with melanoma. https://www.cancerresearch.org/immuno...
In this video, Dr. Jedd D. Wolchok (Memorial Sloan Kettering Cancer Center) addresses:
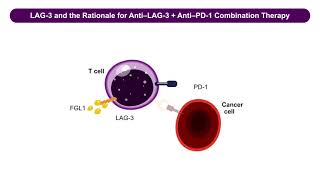
Immune checkpoint inhibitors
Tumorinfiltrating lymphocytes
Cancer immunotherapy side effects
Standard of care for melanoma
Cancer immunotherapy clinical trials
Tumor mutations, such as BRAF and MEK
Oncolytic virus therapy, including TVEC
Combination therapies, such as surgery + immunotherapy, combination immunotherapes, intratumoral injections
How to tell when immunotherapy is working
Treatment regimen decisions, such as length of treatment
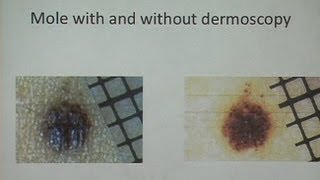
Stages of disease and treatment decisions for melanoma
Overall health of melanoma patient, such as diet and exercise
Dr. Wolchok is a medical oncologist and immunotherapy researcher at Memorial Sloan Kettering Cancer Center (MSK), where he is the chief of Melanoma & Immunotherapeutics Service, the Lloyd J. Old/Virginia and Daniel K. Ludwig Chair in Clinical Investigation, the director of the Parker Institute for Cancer Immunotherapy at MSK, and the associate director of the Ludwig Center for Cancer Immunotherapy. He is also an associate director of the Cancer Research Institute Scientific Advisory Council and chair of the CRI Clinical Trials Network. Dr. Wolchok has been at the forefront of cancer immunotherapy and is renowned for leading the clinical trials that led to the first FDAapproved checkpoint inhibitor immunotherapy, the antiCTLA4 antibody ipilimumab (Yervoy®). He directs a translational research laboratory focused on nextgeneration immunotherapies and combination therapies. https://www.cancerresearch.org/about...
The 2020 CRI Virtual Immunotherapy Patient Summit is part of the Cancer Research Institute's Answer to Cancer Patient Education Program. Established in 1953, the Cancer Research Institute (CRI) is a 501(c)(3) nonprofit organization dedicated to harnessing our immune system’s power to control and potentially cure all cancers. Our mission: Save more lives by fueling the discovery and development of powerful immunotherapies for all types of cancer. To accomplish this, we rely on donor support and collaborative partnerships to fund and carry out the most innovative clinical and laboratory research around the world, support the next generation of the field’s leaders, and serve as the trusted source of information on immunotherapy for cancer patients and their caregivers. https://www.cancerresearch.org
Cancer Research Institute is a registered 501(c)(3) nonprofit under EIN 131837442. Donations are taxdeductible to the fullest extent allowable under the law.