Nitrogen fixation
For more information, log on to
http://shomusbiology.weebly.com/
Download the study materials here
http://shomusbiology.weebly.com/biom...
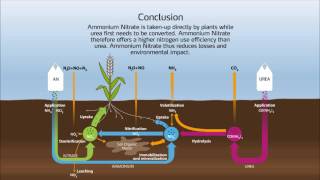
Nitrogen fixation is a process by which nitrogen (N2) in the atmosphere is converted into ammonia (NH3).[1] Atmospheric nitrogen or molecular nitrogen (N2) is relatively inert: it does not easily react with other chemicals to form new compounds. Fixation processes free up the nitrogen atoms from their diatomic form (N2) to be used in other ways.
Nitrogen fixation, natural and synthetic, is essential for all forms of life because nitrogen is required to biosynthesize basic building blocks of plants, animals and other life forms, e.g., nucleotides for DNA and RNA and amino acids for proteins. Therefore nitrogen fixation is essential for agriculture and the manufacture of fertilizer. It is also an important process in the manufacture of explosives (e.g. gunpowder, dynamite, TNT, etc.). Nitrogen fixation occurs naturally in the air by means of lightning.[2][3]
Nitrogen fixation also refers to other biological conversions of nitrogen, such as its conversion to nitrogen dioxide. Microorganisms that can fix nitrogen are prokaryotes (both bacteria and archaea, distributed throughout their respective kingdoms) called diazotrophs. Some higher plants, and some animals (termites), have formed associations (symbioses) with diazotrophs. Biological nitrogen fixation was discovered by the German agronomist Hermann Hellriegel and Dutch microbiologist Martinus Beijerinck. Source of the article published in description is Wikipedia. I am sharing their material. Copyright by original content developers of Wikipedia.
Link http://en.wikipedia.org/wiki/Main_Page