Oxidizing Agent vs Reducing Agent (Oxidant vs Reductant)
An explanation of the differences between oxidizing agents and reducing agents (with examples).
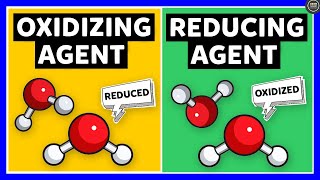
Oxidizing Agent:
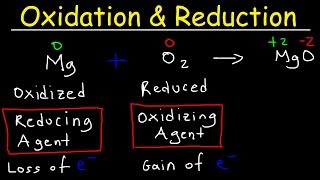
An oxidizing agent is a substance that facilitates oxidation in a chemical reaction.
Oxidation involves the loss of electrons by a chemical species.
The oxidizing agent itself undergoes reduction (gains electrons) during the reaction.
It is often characterized by its ability to accept electrons or cause another substance to lose electrons.
Common examples of oxidizing agents include oxygen (O2, especially in combustion reactions), halogens (e.g., Cl2), and various metal oxides.
Reducing Agent:
A reducing agent is a substance that facilitates reduction in a chemical reaction.
Reduction involves the gain of electrons by a chemical species.
The reducing agent itself undergoes oxidation (loses electrons) during the reaction.
It is typically recognized by its ability to donate electrons or cause another substance to gain electrons.
Common examples of reducing agents include metals (e.g., Zn in the reaction Zn → Zn^2+ + 2e), hydrogen gas (H2), and certain nonmetals.
The key distinction between oxidizing agents and reducing agents lies in their roles during redox reactions: an oxidizing agent promotes oxidation by accepting electrons, while a reducing agent facilitates reduction by donating electrons.
Join this channel to get full access to Dr. B's chemistry guides:
/ @wbreslyn