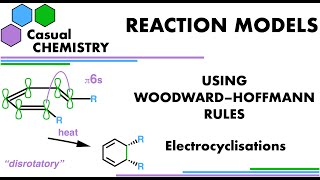
Pericyclic Reactions: Cycloadditions - How to Use Woodward-Hoffmann Rules in Organic Chemistry 1
How to use the WoodwardHoffmann Rules to determine if a pericyclic cycloaddition reaction is allowed under either thermal or photochemical conditions.
#organicchemistry #ochem #orgo #chemistry #stem #science #education
Pericyclic cycloaddition reactions can be identified as a distinct class of pericyclic reaction. During the reaction mechanism, two sigma bonds are formed between two separate pibonded components simultaneously and there is an overall shortening of the total pisystem in the product when compared to the starting material(s). Forming two sigma bonds in this way, more often than not, is a strong thermodynamic driving force (enthalpy mainly) for these pericyclic steps.
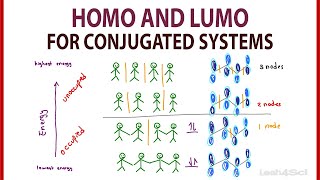
To determine if a specific cycloaddition reaction is allowed by the symmetry of its molecular orbitals under given conditions, the WoodwardHoffmann Rules were developed as a quick way for organic chemists to rationalise experimental observations and predictions. The WoodwardHoffmann Rules have their basis in quantum mechanics and molecular orbital theory (MO theory) and, in cycloadditions, are concerned with analysing the whole set of molecular orbitals associated with a full pi system, including (frequently) conjugated ones. The WoodwardHoffmann Rules are a summary of the results obtained by setting up correlation diagrams that track molecular orbital symmetry conservation in a reaction as a reactant is converted into a product via a transition state. A pericyclic reaction will be symmetry forbidden if the molecular orbital symmetry is not conserved, and this is a result of a large activation energy barrier.
Firstly a threedimensional diagram should be drawn to analyse a specific cycloaddition. A diagram like this defines the molecular orbital overlaps between the pi systems that will become the sigma bonds. The pi systems involved should be identified and labelled with the number of electrons that they contain. It is conventional to add pi or sigma qualifiers as subscripts to the left of the electron count.
It is sensible to work with as few defined pi systems as possible to simplify the WoodwardHoffmann analysis. This is done by remembering to recognise that adjacent pi bond, lone pairs and/or empty porbitals are considered to be conjugated, forming one larger delocalised molecular orbital system, which usually provides a setup for the electrons to lower their total combined energy. In cycloadditions, it is usually possible to analyse using the WoodwardHoffmann rules an arrangement as two larger components interacting at each end. Hence the two sigma bonds that form so all the work for us in defining the facial requirements for the molecular orbitals that must be interacting for the reaction in question. The two components are then assigned as suprafacial or antarafacial depending on each component’s overlap requirement.
The WoodwardHoffmann Rules tell you that: if you count the number of suprafacial components with 4n+2 electrons (where n is an integer) and add that number to antarafacial components with 4n electrons, then the reaction will be thermally allowed when the total sum is an odd number. If the sum is an even number, the reaction is only possible/allowed under photochemical reaction conditions and will not proceed if only heated. Photochemical reactions are performed in the laboratory usually with an ultraviolet light source.
















![How to Memorize Organic Chemistry Reactions and Reagents [Workshop Recording]](https://i.ytimg.com/vi/030FUb25fSs/mqdefault.jpg)










