Pfizer’s Paxlovid Needs Careful Couplings
A video on retrosynthesis in organic chemistry. Paxlovid is the rapidly developed antiviral pharmaceutical agent for the treatment of Covid19 (coronavirus) by the team of scientists at Pfizer. It’s a mixture of two ingredients: Nirmatrelvir and Ritonavir. The mode of action of Nirmatrelvir is thought to be as a covalent inhibitor, with a bond forming between the molecule’s nitrile group and a cysteine residue on the virus protein.
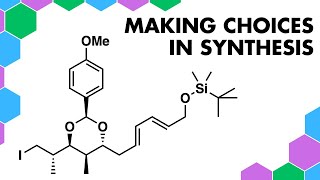
This video is a discussion on organic chemistry synthesis issues when trying to use a disconnection approach (retrosynthetic analysis) on Nirmatrelvir. In particular, there is a focus on chemical coupling reagents for forming amides to minimise risk of epimerisation of alphastereocentres, which can be a problem when using acid chlorides. The video includes a discussion of amide coupling reagents, such as DCC, EDC (EDCI), and HATU these are commonly used in peptide synthesis.
The remainder of the retrosynthesis focuses on three amino acid fragments. These can be synthesised from commercially available starting materials in a few steps using conventional synthesis methods in organic chemistry, including cyclopropanation and exploiting bicyclic structures to impart diastereoselective transformations.
Discovery process discussion from the Pfizer team:
ACS Cent. Sci. 2023, 9, 5, 849–857
https://doi.org/10.1021/acscentsci.3c...
Derek Lowe’s excellent blog posts:
https://www.science.org/content/blog...
https://www.science.org/content/blog...
Hanessian dianion chemistry:
Tet. Lett., 1998, 39, 5887
https://doi.org/10.1016/S00404039(98...
#chemistry #organicchemistry #science