pH and Acid Base Balance | Biochemistry
pH and Acid Base Balance | Biochemistry
Like this video?
Sign up now on our website at https://www.DrNajeebLectures.com to access 800+ Exclusive videos on Basic Medical Sciences & Clinical Medicine. These are premium videos (NOT FROM YOUTUBE). All these videos come with English subtitles & download options. Sign up now! Get Lifetime Access for a onetime payment of $99 ONLY!
Sign up now on our website at https://members.drnajeeblectures.com/'>https://members.drnajeeblectures.com/
Why sign up for premium membership? Here's why!
Membership Features for premium website members.
1. More than 800+ Medical Lectures.
2. Basic Medical Sciences & Clinical Medicine.
3. Mobilefriendly interface with android and iOS apps.
4. English subtitles and new videos every week.
5. Download option for offline video playback.
6. Fanatic customer support and that's 24/7.
7. Fast video playback option to learn faster.
8. Trusted by over 2M+ students in 190 countries.
▬▬▬▬▬▬▬▬▬▬ Contents of this video ▬▬▬▬▬▬▬▬▬▬
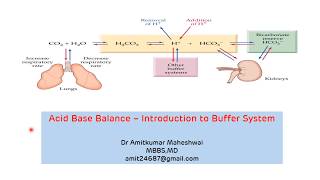
The body's balance between acidity and alkalinity is referred to as acidbase balance. The blood's acidbase balance is precisely controlled because even a minor deviation from the normal range can severely affect many organs. The body uses different mechanisms to control the blood's acidbase balance.
To maintain homeostasis, the human body employs many physiological adaptations. One of these is maintaining an acidbase balance. In the absence of pathological states, the pH of the human body ranges between 7.35 to 7.45, with the average at 7.40. Why this number? Why not a neutral number of 7.0 instead of a slightly alkaline 7.40? A pH at this level is ideal for many biological processes, one of the most important being the oxygenation of blood. Also, many of the intermediates of biochemical reactions in the body become ionized at a neutral pH, which causes the utilization of these intermediates to be more difficult.
A pH below 7.35 is an acidemia, and a pH above 7.45 is an alkalemia. Due to the importance of sustaining a pH level in the needed narrow range, the human body contains compensatory mechanisms. This discussion intends to impart a basic understanding of acidbase balance in the body while providing a systematic way to approach patients who present with conditions causing alterations in pH.
The human body experiences four main types of acidbased disorders: metabolic acidosis, metabolic alkalosis, respiratory acidosis, and respiratory alkalosis. If one of these conditions occurs, the human body should induce a counterbalance in the form of an opposite condition. For example, if a person is experiencing a metabolic acidemia, their body will attempt to induce a respiratory alkalosis to compensate. It is rare for the compensation to make the pH completely normal at 7.4. When using the term acidemia or alkalemia, one is denoting that overall the pH is acidic or alkalotic, respectively. While not necessary, it can be useful to employ this terminology to distinguish between individual processes and the overall pH status of the patient since multiple imbalances can happen at the same time.
Join this channel to get access to the perks:
Sign up now on our website at https://members.drnajeeblectures.com/'>https://members.drnajeeblectures.com/
Follow us on Facebook: / drnajeeb
Follow us on Instagram: / drnajeeblectures