Physiological Buffer system acid base balance | Renal system physiology lecture
Physiology lecture on renal system physiology details how buffers act, what is dissociation constant and what is Henderson Hasselback equation which explains when a buffer will be most effective. Also learn about bicarbonate buffer system, phosphate buffer system and ammonia buffer system.
Buy concept based physiology notes here:
Download
Android app for Physiology notes here: https://play.google.com/store/apps/de...
IOS app: https://apps.apple.com/in/app/myinsti... (use org code:ezlep)
Website: http://www.physiologyopen.com/
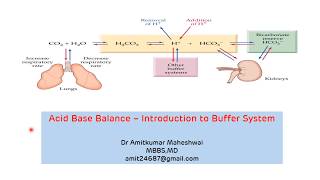
Strong acids and bases dissociate almost completely in an aqueous solution into respective ions and hence add H+ ion and hydroxyl ions into solution causing a change in pH.
Buffer is any substance which binds H+ ions reversibly and prevents the change in pH in case there is addition of acids or bases into a solution. Weak acids which do not completely ionise when dissolved in water behave as buffers. Fundamentally the weak acid acts as proton donor and its conjugate base acts as a proton acceptor.
Now when can be a buffer most effective and how one buffer differs from other buffers ?
This depends on the dissociation characteristics of any acid i.e its tendency to release hydrogen ions into a solution. The dissociation characteristic of the acids is studied using equilibrium constant or dissociation constant. But how this dissociation constants helps us in understanding the characteristics of the buffer and when they are most effective ? That is better understood with Henderson Hasselbalch equation such that we are solving for H+.
Watch our other popular playlists:
CNS physiology: • Central nervous system | CNS Physiolo...
Cardiovascular physiology: • Cardiovascular physiology mbbs 1st ye...
Respiratory physiology: • Respiratory system physiology mbbs 1s...
General Physiology: • General physiology mbbs 1st year lect...
Blood physiology: • Blood physiology, pathology, pharmaco...
Renal Physiology: • Renal system physiology lectures | 1s...
Endocrine physiology: • Endocrine system | Physiology lecture...
Buy our Practical Physiology book here:
Amazon: https://www.amazon.in/PracticalPhysi...
Flipkart: https://www.flipkart.com/practicalph...
Chapters:
00:00 Introduction
00:58 What is a buffer
03:22 Dissociation constant
04:57 Henderson Hasselbach equation
07:45 Physiological buffers
#physiology
#1styearMBBS
#MBBS1styear
#physiologylectures
#renalphysiology
#physiologyopen
#humanphysiology