Polar Molecules Tutorial: How to determine polarity in a molecule
This video looks at how to determine polarity in a molecule by understanding how the bond polarities, molecule shape, and outside atoms influence polarity using bond polarity vector addition. This includes a flow chart that guides you through the various decisions needed to determine if a molecule is polar or not.
Link for PT with electronegativities: https://drive.google.com/file/d/0B03U...
CC Academy videos are easy 101 crash course tutorials for step by step Chemistry help on your chemistry homework, problems, and experiments:
Stoichiometry Tutorial, step by step
Types of Chemical Reactions: How to classify five basic reaction types
Solution Stoichiometry
Orbitals the Basics: Atomic Orbitals Tutorial
Hybrid Orbitals Explained
Polar Molecules Tutorial: How to determine polarity in a molecule
Metallic Bonding and Metallic Properties Explained
Covalent Bonding Tutorial
Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
Metric Unit Prefix Conversions: How to Convert Metric System Prefixes
Metric unit conversions shortcut: fast, easy howto with examples
Mole Conversions Tutorial: how to convert mole mass, mole particle, mass particle problems
Frequency, Wavelength, and the Speed of Light
The Bohr Model of the Atom and Atomic Emission Spectra
What is Heat: A brief introduction at the particle level
Rutherford's Gold Foil Experiment
Unit Conversion Using Dimensional Analysis Tutorial
What is Fire: Combustion Reaction Tutorial
Quantum Numbers Tutorial
Electron Configurations Tutorial and How to Derive Electron Configurations from the Periodic Table
Concentration and Molarity Explained
Heating Curves Tutorial
Naming Ionic Compounds
Limiting Reactant Tutorial
Gas density and PV=nRT, the ideal gas law
Surface Tension What is it, how does it form, what properties does it impart
Wikipedia: In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. A polar molecule with two or more polar bonds must have a geometry which is asymmetric in at least one direction, so that the bond dipoles do not cancel each other.
While the molecules can be described as "polar covalent", "nonpolar covalent", or "ionic", this is often a relative term, with one molecule simply being more polar or more nonpolar than another. However, the following properties are typical of such molecules.
A molecule is composed of one or more chemical bonds between molecular orbitals of different atoms. A molecule may be polar either as a result of polar bonds due to differences in electronegativity as described above, or as a result of an asymmetric arrangement of nonpolar covalent bonds and nonbonding pairs of electrons known as a full molecular orbital.
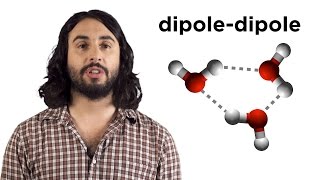
The water molecule is made up of oxygen and hydrogen, with respective electronegativities of 3.44 and 2.20. The dipoles from each of the two bonds (red arrows) add together to make the overall molecule polar.
A polar molecule has a net dipole as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. Water (H2O) is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. The dipoles do not cancel out resulting in a net dipole. Due to the polar nature of the water molecule itself, polar molecules are generally able to dissolve in water. Other examples include sugars (like sucrose), which have many polar oxygen–hydrogen (−OH) groups and are overall highly polar.
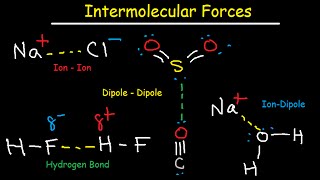
If the bond dipole moments of the molecule do not cancel, the molecule is polar. For example, the water molecule (H2O) contains two polar O−H bonds in a bent (nonlinear) geometry. The bond dipole moments do not cancel, so that the molecule forms a molecular dipole with its negative pole at the oxygen and its positive pole midway between the two hydrogen atoms.
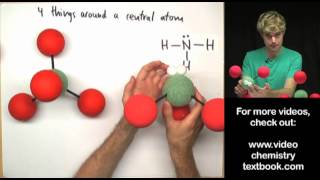
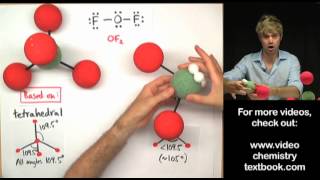
The hydrogen fluoride, HF, molecule is polar by virtue of polar covalent bonds – in the covalent bond electrons are displaced toward the more electronegative fluorine atom. Ammonia, NH3, molecule the three N−H bonds have only a slight polarity (toward the more electronegative nitrogen atom). The molecule has two lone electrons in an orbital, that points towards the fourth apex of the approximate tetrahedron, (VSEPR).
When comparing a polar and nonpolar molecule with similar molar masses, the polar molecule in general has a higher boiling point, because the dipole–dipole interaction between polar molecules results in stronger intermolecular attractions. One common form of polar interaction is the hydrogen bond, which is also known as the Hbond.