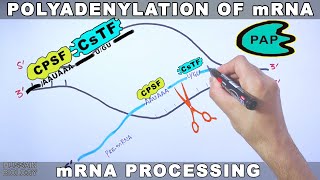
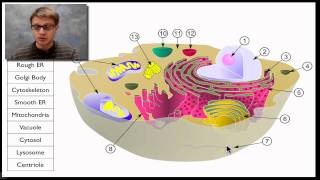
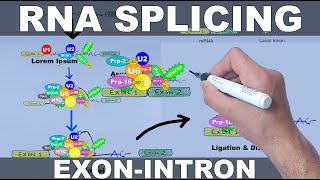
Polyadenylation of mRNA (poly a tail)
For more information, log on to
http://shomusbiology.weebly.com/
Download the study materials here
http://shomusbiology.weebly.com/biom...
Polyadenylation is the addition of a poly(A) tail to an RNA molecule. The poly(A) tail consists of multiple adenosine monophosphates; in other words, it is a stretch of RNA that has only adenine bases. In eukaryotes, polyadenylation is part of the process that produces mature messenger RNA (mRNA) for translation. It, therefore, forms part of the larger process of gene expression.
The process of polyadenylation begins as the transcription of a gene finishes, or terminates. The 3'most segment of the newly made RNA is first cleaved off by a set of proteins; these proteins then synthesize the poly(A) tail at the RNA's 3' end. In some genes, these proteins may add a poly(A) tail at any one of several possible sites. Therefore, polyadenylation can produce more than one transcript from a single gene (alternative polyadenylation), similar to alternative splicing.[1]
The poly(A) tail is important for the nuclear export, translation, and stability of mRNA. The tail is shortened over time, and, when it is short enough, the mRNA is enzymatically degraded.[2] However, in a few cell types, mRNAs with short poly(A) tails are stored for later activation by repolyadenylation in the cytosol.[3] In contrast, when polyadenylation occurs in bacteria, it promotes RNA degradation.[4] This is also sometimes the case for eukaryotic noncoding RNAs.[5]
The polyadenylation machinery in the nucleus of eukaryotes works on products of RNA polymerase II, such as precursor mRNA. Here, a multiprotein complex (see components on the right) cleaves the 3'most part of a newly produced RNA and polyadenylates the end produced by this cleavage. The cleavage is catalysed by the enzyme CPSF[11] and occurs 1030 nucleotides downstream of its binding site.[16] This site is often the sequence AAUAAA on the RNA, but variants of it that bind more weakly to CPSF exist.[17] Two other proteins add specificity to the binding to an RNA: CstF and CFI. CstF binds to a GUrich region further downstream of CPSF's site.[18] CFI recognises a third site on the RNA (a set of UGUAA sequences in mammals[19][20][21]) and can recruit CPSF even if the AAUAAA sequence is missing.[22][23] The polyadenylation signal the sequence motif recognised by the RNA cleavage complex varies between groups of eukaryotes. Most human polyadenylation sites contain the AAUAAA sequence,[18] but this sequence is less common in plants and fungi.[24]
The RNA is typically cleaved before transcription termination, as CstF also binds to RNA polymerase II.[25] Through a poorlyunderstood mechanism (as of 2002), it signals for RNA polymerase II to slip off of the transcript.[26] Cleavage also involves the protein CFII, though it is unknown how.[27] The cleavage site associated with a polyadenylation signal can vary up to some 50 nucleotides.[28]
When the RNA is cleaved, polyadenylation starts, catalysed by polyadenylate polymerase. Polyadenylate polymerase builds the poly(A) tail by adding adenosine monophosphate units from adenosine triphosphate to the RNA, cleaving off pyrophosphate.[29] Another protein, PAB2, binds to the new, short poly(A) tail and increases the affinity of polyadenylate polymerase for the RNA. When the poly(A) tail is approximately 250 nucleotides long the enzyme can no longer bind to CPSF and polyadenylation stops, thus determining the length of the poly(A) tail.[30][31] CPSF is in contact with RNA polymerase II, allowing it to signal the polymerase to terminate transcription.[32][33] When RNA polymerase II reaches a "termination sequence" (TTATT on the DNA template and AAUAAA on the primary transcript), the end of transcription is signaled.[34] The polyadenylation machinery is also physically linked to the spliceosome, a complex that removes introns from RNAs.[23] Source of the article published in description is Wikipedia. I am sharing their material. Copyright by original content developers of Wikipedia.
Link http://en.wikipedia.org/wiki/Main_Page