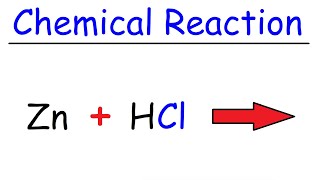
Predict the Products of the Reaction for Mg + HCl (Magnesium + Hydrochloric acid)
Predict the Products of the Reaction for Mg + HCl (Magnesium + Hydrochloric acid)
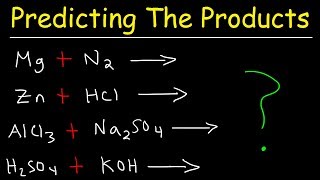
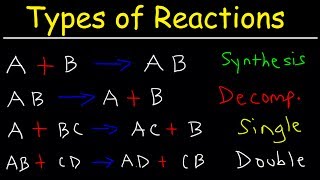
To predict the products of the chemical reaction between Mg and HCl (Magnesium and Hydrochloric acid), we first need to recognize the type of reaction that will take place.
Predicting the Products of Chemical Reactions: • Predicting the Products of Chemical R...
Types of Reactions: • Major Types of Chemical Reactions (Ex...
Predicting Reactions Playlist: • Types of Reactions and Predicting Pro...
Because Mg is a metal and HCl is an acid, we have a single displacement reaction. Based on this information, we can predict that the products will be Magnesium chloride (MgCl2) and Hydrogen gas (H2).
This reaction is also an example of a metalacid reaction, where the metal replaces the hydrogen in the acid, resulting in the formation of a salt (magnesium chloride in this case) and the release of hydrogen gas. This allows us to figure out the products of the reaction between Mg and HCl.