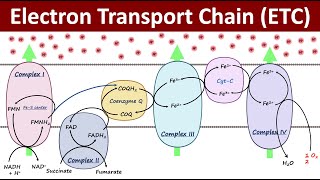
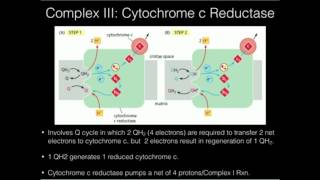
Q Cycle (ELECTRON TRANSPORT CHAIN)
This video comprehensively explains the intriguing details pertaining to the Q cycle.
In the Q cycle mechanism, protons are carried across the membrane as hydrogen atoms on the hydroxyl groups of ubiquinol. However, the sites where ubiquinol is oxidized at center P and ubiquinone is reduced at center N are not freely accessible to the bulk aqueous phase at the membrane surface. Consequently, the linkage of proton chemistry to electron transfer requires mechanisms for moving protons to and from the aqueous phase and the hydrophobic sites of quinol and quinone redox reactions. When ubiquinol reduces the Rieske protein, an electron is transferred to the iron–sulfur cluster, while a proton from the quinol hydroxyl group protonates the imidazole nitrogen on His181 of the Rieske protein. The Rieske protein acts as a hydrogen carrier and releases the proton to the aqueous phase when it moves proximal to cytochrome c1 and is oxidized. The second proton from ubiquinol is transferred to Glu272 and then to a propionate of the cytochrome b heme as an electron is transferred to the bL heme. From the crystal structure of the yeast enzyme it appears that this proton can then access the aqueous surface from the bL heme via a conserved arginine (Arg79). In this manner, two protons are released from center P with minimal charge separation as the two electrons are divergently transferred from the quinol to the highpotential and lowpotential electron acceptors.