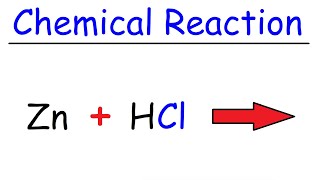
Reaction of Zinc and Hydrochloric acid
In this video, we'll be exploring the reaction between zinc metal and hydrochloric acid. The equation for this reaction is Zn + HCl → ZnCl2 + H2. As the reaction proceeds, you'll notice bubbles forming, which is hydrogen gas (H2) being released. We can capture these bubbles and test them by lighting a flame to see the characteristic pop sound of burning hydrogen gas.
In the reaction, zinc chloride, which is one of the products of the reaction, stays dissolved in water. Zinc chloride is an aqueous solution that remains dissolved in water due to its watersoluble nature. This reaction is a classic example of a single replacement reaction, where a more reactive metal, in this case, zinc, replaces a less reactive metal, hydrogen.