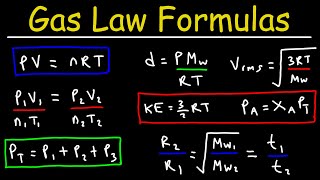
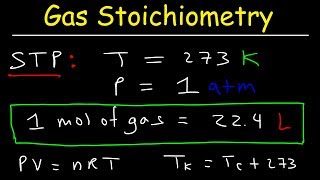
Real vs Ideal Gases
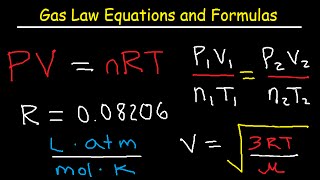
Ideal gases don't really exist. But under Low Pressure and at High Temperatures many gases are close to ideal. That makes the Ideal Gas Law really usefully in many real world situations. Most of the gases in air are close to ideal at STP (except CO2).
Awesome visualization app: https://phet.colorado.edu/en/simulati...
Here’s why:
1. For the ideal gas law we assume that molecules don’t take up any space. But they really do. This doesn’t matter as much at low pressure where they are spread out. We have mostly empty space. But increase the pressure, the volume occupied by molecules starts to matter.
2. For Ideal Gases we assume that molecules don’t attract or repel each other. This works well at higher temperatures. Molecules are moving fast so it’s more difficult to interact. But lower the temperature, now they can interact. Dipoledipole, Van der Waals forces, or even hydrogen bonding.
So in short gases are ideal at lower pressures and higher temperatures.
The good news is we have equations to deal with the other situations, they are just a bit more complicated. For example, Van der Waals equation (https://en.wikipedia.org/wiki/Van_der...) or
the PengRobinson Equation of State ( https://en.m.wikipedia.org/wiki/Cubic...)
Join this channel to get full access to Dr. B's chemistry guides:
/ @wbreslyn