Get free YouTube views, likes and subscribers
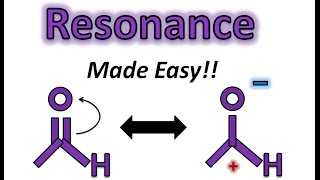
Resonance Structures of O3 Ozone
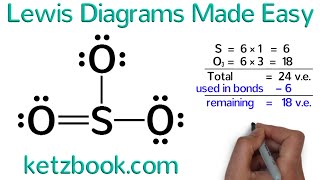
Ozone molecules are three oxygen atoms bonded in succession, they are NOT in a ring.
The middle oxygen, according to the Lewis Structure, needs one double bond and one single bond ... but because the double can come from either direction (left or right), there are two valid Lewis Structures.
Each of these is separately called a "resonance structure" and the true structure of Ozone is somewhere in between these two. Here I draw both resonance structures and a resonance hybrid as well.
Check me out: http://www.chemistnate.com
Recommended