Retrosynthesis 1 - Organic Chemistry
A quick introductory level retrosynthesis, followed by an explanation of some of the relevant mechanisms for a proposed synthesis.
#chemistry #organicchemistry #ochem #orgo #retrosynthesis
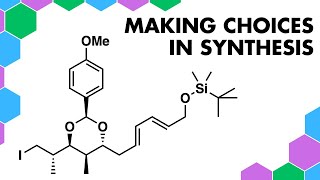
I have picked a random molecule to explain a retrosynthesis and my thoughts on a retrosynthetic analysis. I have taken quite a classical, old school approach in this video. Techniques described are those commonly found in books describing a "Disconnection Approach" such as the common education textbook by Stuart Warren from the University of Cambridge.
I'm hoping this video helps with people learning about building together their ideas of synthesis in organic chemistry. Importantly here is that there is one functional group that is more reactive than the others. It isn't a smart plan to carry a reactive group through loads of steps in a forward synthesis so I am showing that it should be disconnected quickly when identified. In this case, the target molecule has a free thiol (this functional group is sometimes known by the name "mercaptan").
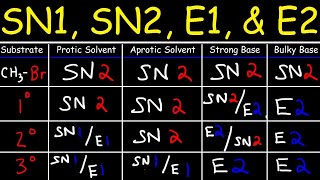
In a classical organic synthesis, a thiol is made from an SN2 type process as described using thiourea, followed by an alkaline workup. So by default you need to engineer a good synthesis by setting up everything in favour of doing an SN2 reaction, for example by in the first instance by making sure you have a good leaving group on a saturated sp3 carbon centre.
In general for setting up an SN2 substitution reaction at a saturated carbon atom, a really good approach is to first form an alcohol because there are loads of reactions that readily generate alcohols incidentally when forming carboncarbon bonds. In this video, there is a FriedelCrafts acylation reaction but another key workhorse reaction in organic synthesis would be the the aldol reaction.
There are loads of ways of turning alcohols into alkyl halides, or alkyl(leaving groups), but here I present a mechanistic explanation for how using phosphorus tribromide (PBr3) works. Similarly, phosphorus trichloride would work for making the alkyl chloride instead, as would thionyl chloride. If you wanted to make the alkyl iodide, you could use triphenylphosphine with elemental iodine (PPh3, I2). These substitution reaction can also be set up my making a good leaving group from the alcohol such as the tosylate or the mesylate (and many many other alternatives).
Once you have a leaving group installed, a good way of making a thiol (mercaptan) is to treat the alkyl halide (halogenoalkane/haloalkane) with thiourea. The soft nucleophile on the sulfur can do an SN2 reaction, and the intermediate hydrolysed to the free thiol by treatment with sodium hydroxide. This method is particularly reliable to prevent multiple substitution reactions occurring given that the product thiol is itself a good SN2 nucleophile. This is a handy disconnection for this oxidation state of sulfur in any planned retrosynthesis.
The FriedelCrafts acylation is under normal para selectivity dictated mainly from the pi electon donating group (the Nattached amide). Getting the aromatic benzene ring to react with an electrophile requires, as usual, a powerful electrophile in this case an acylium ion works for this.









![How to Memorize Organic Chemistry Reactions and Reagents [Workshop Recording]](https://i.ytimg.com/vi/030FUb25fSs/mqdefault.jpg)