Retrosynthesis 12 PKI-166 - Organic Chemistry
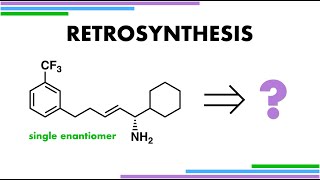
Retrosynthetic analysis of PKI166 – a EGFR tyrosine kinase inhibitor that inhibits pancreatic cancers. The retrosynthesis uses a cheap chiral pool starting material and involves the construction of the bicyclic nitrogen heterocycle from scratch.
#orgo #organicchemistry #ochem #synthesis #science
The retrosynthesis begins by separation of the aromatic component of the molecule from the single stereocentre at the CN bond. This disconnection is sensible as an amine can be attached to an aromatic ring when it acts as the nucleophile in an SNAr substitution reaction. The amine nucleophile is actually cheap and readily available from the chiral pool as 1phenylethylamine. 1Phenylethylamine is obtained as a single enantiomer by chiral resolution with Lmalic acid, which occurs naturally as part of the citric acid cycle and the Calvin cycle in biochemistry. A racemic mixture of 1phenylethylamine is first synthesised from the reductive amination of acetophenone with ammonia. When this racemic mixture is treated with the single enantiomer of malic acid, two diastereomeric salts form. The salt formed with D1phenylethylamine crystallises out of solution, whereas the salt formed with L1phenylethylamine stays in solution allowing for easy physical separation. The malic acid salts can then be treated with a base to return the free amine and the malic acid resolving agent washed away. Single enantiomers of 1phenylethylamine are themselves used frequently in organic synthesis as chiral resolving agents (for other chiral resolutions).
Turning to the nitrogen heterocycle, we can envisage creating each half – the pyrrolelike half and the pyridonelike half – separately by ring closing reactions. Firstly, the aryl chloride required for the SNAr substitution with 1phenylethylamine must be disconnected first as it is reactive. These 2chloropyridine type structures can be easily constructed from the parent pyridone using a deoxygenating reagent, such as phosphoryl chloride (POCl3). The 6membered ring part of this nitrogen heterocycle can be disconnected between the two nitrogens and extracting the carbon which is of the same oxidation level of an amide. The bonding pattern can therefore be constructed by condensing a reagent such as formamide (HCONH2) or methyl formate (HCOOMe) between two nitrogen centres. This disconnection leaves behind a trisubstituted pyrrole (2,4,5substituted). This type of pyrrole ring can be synthesised from a variety of methods exploiting either the inherent reactivity of the pyrrole heterocycle or by de novo construction of the aromatic heterocycle from a linear precursor, which is proposed in this video.
When the pyrrole ring is disconnected between the 1 (N) and 2positions, the linear precursor for cyclisation will be a 1,4diX (1,4difunctionalised) carbonyl species, which will form the 5membered heterocycle. 1,4diX compounds are often synthesised by using Umpolung chemistry (reversed polarity). One possible option would be to use an alphahalocarbonyl reagent that incorporates the C2 and C3 from the target pyrrole, and target it’s soft electrophilic centre with a soft nucleophile. An appropriate soft nucleophile would be based on a 1,3dicarbonyl species (based on a malonate) which would also bring in the correct oxidation levels at both C5 and the branched position coming off C4. An anion of a 1,3dicarbonyl species would form the soft nucleophile at the position that would become the C4 position in the target pyrrole.
The alphahalo carbonyl required for the Umpolung chemistry above can be simply constructed by monobromination, for example, of the 4’methoxyacetophenone (4acetylanisole). This is compound is pretty cheap in itself, but could be synthesised by FriedelCrafts acylation of anisole. The standard nucleophilic reactivity of a benzene ring bearing a pi electron donating group would ensure that the major product of such a FriedelCrafts acylation would be substitution in the para position.