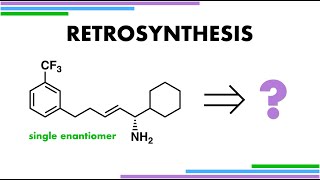
Retrosynthesis with Aldols
A classical disconnection approach for a retrosynthesis of this molecule with a benzyl protecting group on a basesensitive aldol product.
More retrosynthesis videos available at this playlist:
• Retrosynthesis
Retrosynthetic analysis identifies disconnection of the benzyl ether as a protecting group for the hydroxyl group of an aldol product. A Williamson ether synthesis is not possible to form this CO bond due to a risk of retroaldol fragmentation under strongly basic conditions. A more mild trichloroacetimidate reagent (TCA) can be used to benzylate under mild acidic conditions instead.
The next disconnection will come from identifying a 1,3difunctional relationship (1,3diX) which is best formed by enolate chemistry using an aldol reaction. The lithium enolate formed by reaction of ethyl acetate with LDA is appropriate for this transformation.
The aldehyde required for this aldol reaction also has a 1,3difunctional relationship between the aldehyde carbonyl and an alkene. To use enolate chemistry and aldol reactions again, a functional group interconversion must be used. The alkene can be formed by elimination reaction from an alcohol. An E2 elimination reaction can be done by first converting the hydroxyl group into a tosylate leaving group, and then treating the tosylate species with a nonnucleophilic base such as potassium tertbutoxide (KOtBu).
The final intermediate in the retrosynthesis can also be disconnected by aldol chemistry back to isobutyraldehyde. Isobutyraldehyde will be treated with a reversible base to cause the aldol selfreaction between the enolate and the aldehyde forms of this molecule, as nucleophile and electrophile, respectively.
#organicchemistry #retrosynthesis #chemistry