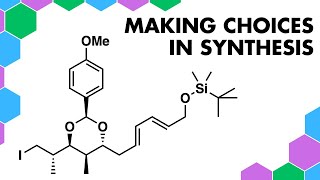
Retrosynthesis with Hypervalent Iodine
Disconnecting this tricyclic ring molecule using hypervalent iodine reagents. Also showcasing Umpolung chemistry and the DielsAlder reaction.
Reference: Desymmetrisation of phenols by an asymmetric Stetter reaction (Rovis)
J. Am. Chem. Soc. 2006, 128, 8, 2552–2553
https://doi.org/10.1021/ja058337u
The cyclohexene ring can be targeted first in the retrosynthetic analysis of this molecule to give an electronrich diene and electronpoor dienophile with the correct molecular orbital coefficients to lead to the required regioselectivity. The diastereoselectivity for the DielsAlder reaction comes from going through the usual exo transition state.
This leaves a 1,4diX disconnection for the diketone, which can be achieved by Stetter reaction. A thiazolium salt catalyst can form a nucleophilic ylid when it is treated with a mild base. The nucleophile can attack the aldehyde intermediate in the retrosynthesis, and after a few proton transfers will form the Breslow intermediate, essentially an enamine generated in situ. The enamine is held right next to the alpha,betaunsaturated ketones and so a Michael addition (conjugate addition) is easy to form the 6,5fused ring system.
The Michael acceptor intermediate can be made by dearomatisation of a phenol using PIDA (also known as BAIB or PhI(OAc)2 ). This hypervalent iodine reagent is a strong oxidising agent and makes the aromatic ring of the phenol electrophilic. When the benzene ring is attacked by a nucleophile, a new C=O double bond (carbonyl) is formed at the same time as the loss of two leaving groups in iodobenzene and an acetate anion.
In the video, hypervalent iodine is also used for the oxidation of an alcohol to an aldehyde as the DessMartin oxidation. The mechanism for the oxidation of the alcohol by DessMartin periodinane (DMP) is given.
#chemistry #organicchemistry #science