Soap Micelles Formation - Science
MICELLES
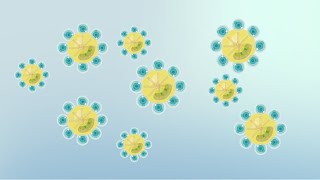
Soaps are molecules having two ends with differing properties, one end is hydrophilic, that is, it dissolves in water, while the other end is hydrophobic, that is, it dissolves in hydrocarbons, and doesn't dissolve in water. Since the hydrophobic 'tail' of the soap molecules will not be soluble in water, the soap molecules will align along the surface of water with the hydrophilic end (ionic end) in water and the hydrocarbon 'tail' protruding out of water. Inside water, the soap molecules are orientated such that the hydrocarbon portion is out of the water. This is achieved by forming clusters of soap molecules in which the hydrophobic tails group at the interior of the cluster and the ionic ends protrude on to the surface of the cluster.
This formation is called a micelle. The mechanism of soap cleansing is attributed to these micelles. Soap in the form of micelles is capable of cleaning, since the oily dirt will be collected in the centre of the micelle due to the hydrophobic tails. The micelles stay in solution as a colloid and will not come together to precipitate because of ionion repulsion. Thus, the dirt held along with the micelles is also easily rinsed away. The soap solution appears cloudy since the micelles are large enough to scatter light.