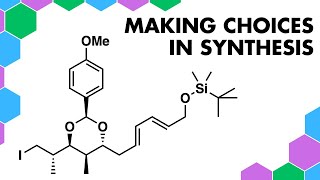
Staudinger Reactions - Bioothogonal before Click Chemistry
The original bioorthogonal chemistry using azides and their reaction phosphines to form azaylids, which can do bioconjugation reactions and other useful transformations in organic synthesis.
The azaylid (aminophosphorane) generated can react directly with water in a hydrolysis reaction to give an amine product. The reduction of the azide to the amine under these conditions is mild and tolerant of many other functional groups, whereas a hydrogenation or borohydride reduction might not be. The only byproduct it triphenylphosphine oxide which is inert, separable and provides a strong enthalpy driving force.
The azaylid can also react with carbonyl functional groups. If it reacts with a ketone or an aldehyde, you make the imine product, that can be useful in general synthesis of nitrogen containing molecules, including heterocycles.
#chemistry #organicchemistry #science