The Brain's Hunger/Satiety Pathways and Obesity Animation
(USMLE topics, neurobiology) The appetite pathway in the brain, leptin, and pathology of obesity.
Purchase a license to download a nonwatermarked version of this video on AlilaMedicalMedia(dot)com
Check out our new Alila Academy AlilaAcademy(dot)com complete video courses with quizzes, PDFs, and downloadable images.
©Alila Medical Media. All rights reserved.
Voice by: Ashley Fleming
All images/videos by Alila Medical Media are for information purposes ONLY and are NOT intended to replace professional medical advice, diagnosis or treatment. Always seek the advice of a qualified healthcare provider with any questions you may have regarding a medical condition.
Food intake and energy expenditure must be balanced to maintain a healthy body weight. This balance is kept by the central nervous system, which controls feeding behavior and energy metabolism.
Several brain systems are involved, including the brainstem which receives neuronal inputs from the digestive tract, and the hypothalamus which picks up hormonal and nutritional signals from the circulation. They also interact with the reward and motivation pathways, which drive foodseeking behavior.
The arcuate nucleus, ARC, of the hypothalamus: two groups of neurons, with opposing functions, in the ARC: the appetitestimulating neurons expressing NPY and AGRP peptides, and the appetitesuppressing neurons producing POMC peptide.
Neurons of the ARC project to other nuclei of the hypothalamus, of which the paraventricular nucleus, PVN, is most important. PVN neurons further process the information and project to other circuits outside the hypothalamus, thus coordinating a response that controls energy intake and expenditure.
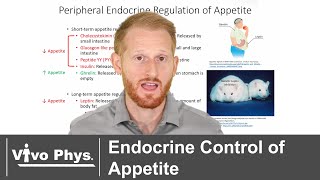
Shortterm regulation of feeding is based on how empty or how full the stomach is, and if there are nutrients in the intestine. In the fasting state, an empty stomach sends stretch information to the brainstem, signaling hunger. It produces a peptide called ghrelin, which acts on the arcuate nucleus to stimulate feeding. Ghrelin also acts directly on the PVN to reduce energy expenditure.
Upon food ingestion, distension of the stomach is perceived by the brainstem as satiety. Several other gut peptides are released from the intestine and act on the hypothalamus and other brain areas to suppress appetite and increase energy expenditure.
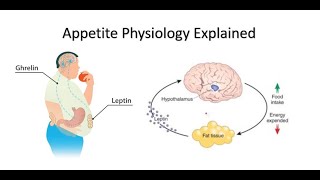
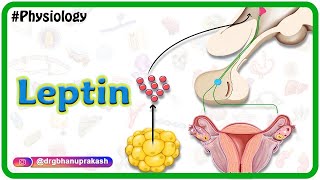
Longterm regulation takes cues from the amount of body fat: low body fat content encourages feeding and energy preservation, while high body fat suppresses appetite and promotes energy expenditure. Two hormones are involved: leptin and insulin.
Insulin is a hormone produced by the pancreas and is released into the bloodstream upon food ingestion, when blood glucose starts to rise. Leptin is a hormone secreted by adipose tissues in a process dependent on insulin. The amount of circulating leptin in the plasma is directly proportional to the body fat content. Increased leptin levels in the blood signal to the brain that the body has enough energy storage, and that it has to stop eating and burn more energy. Leptin and insulin seem to work together on hypothalamic nuclei, as well as other brain areas, to inhibit food intake and increase energy expenditure.
Obesity results from the dysregulation of feeding behaviors and energy metabolism. Obesity is associated with chronic low leptin activities, which trick the brain into thinking that the body is always starved. This leads to overeating and excessive energy storage as fats.
The major lifestyle factor is a highfat, energyrich diet. In an early stage of highfatdiet–induced obesity, increased amounts of saturated fatty acids cross the blood brain barrier and provoke an inflammatory response in hypothalamic neurons. Inflammation induces stress in these neurons, blunting their response to leptin. This is known as leptin resistance. Leptin levels are high, but because the cells cannot react to leptin, the brain interprets it as low and triggers the starvation response.
Genetic factors include mutations in the leptin gene itself, or in one of the numerous downstream genes that are required for leptin action in various pathways. Leptin deficiency due to gene mutations is very rare. More common are mutations in the downstream genes, which render a certain pathway irresponsive to leptin.
A major risk factor for childhood obesity is maternal obesity and mother’s highfatdiet during pregnancy and lactation. A maternal diet rich in saturated fats can cause inflammation in the infant’s hypothalamus. It may also prime the reward pathways in infants, influencing their food choice toward energyrich foods.